AI-Powered
Quality Management System
Close out deviations and CAPAs faster while simplifying your compliance needs. Our QMS provides a single source of truth for all your quality processes, from risk and audit management to change control and CAPAs.
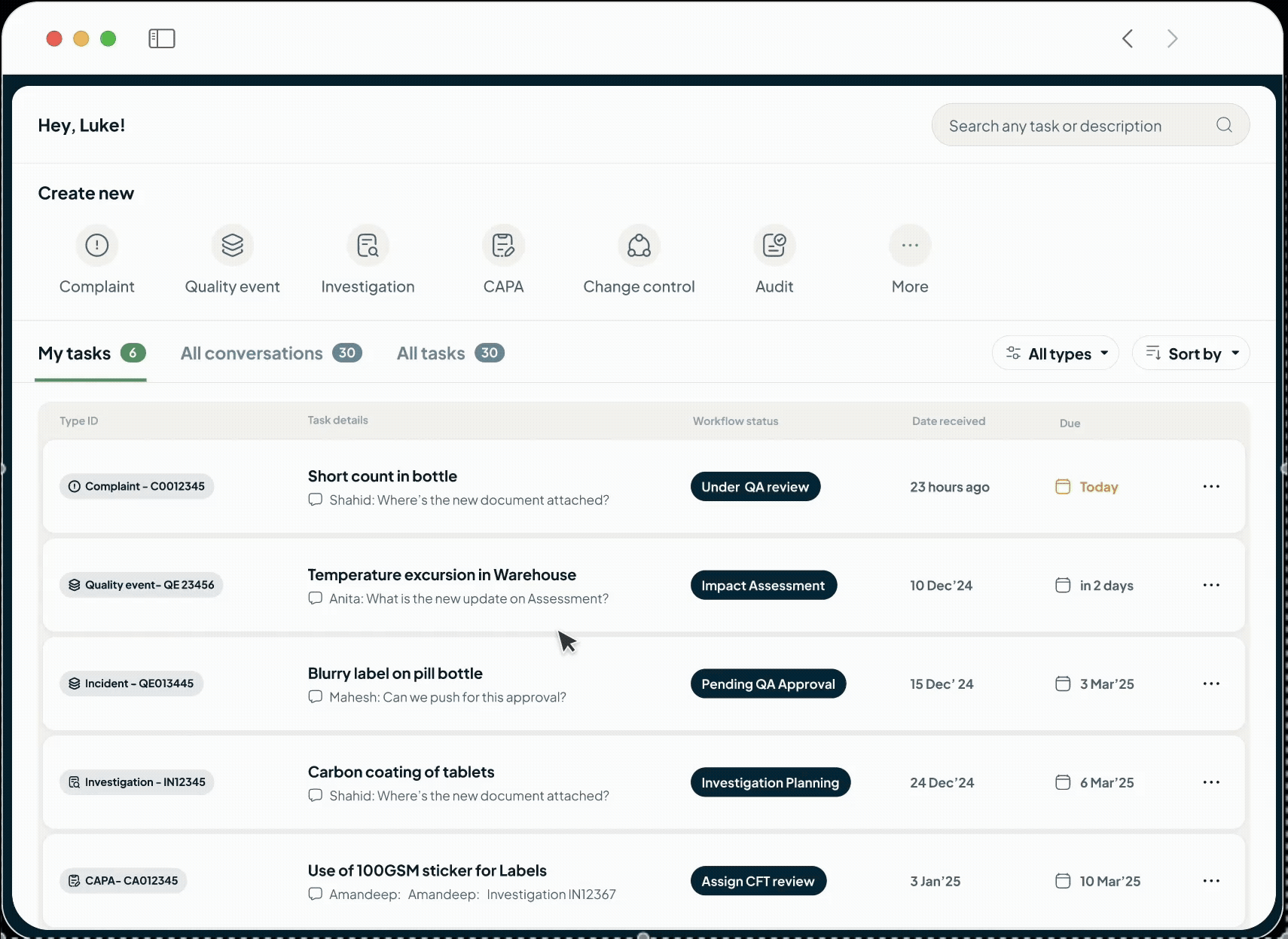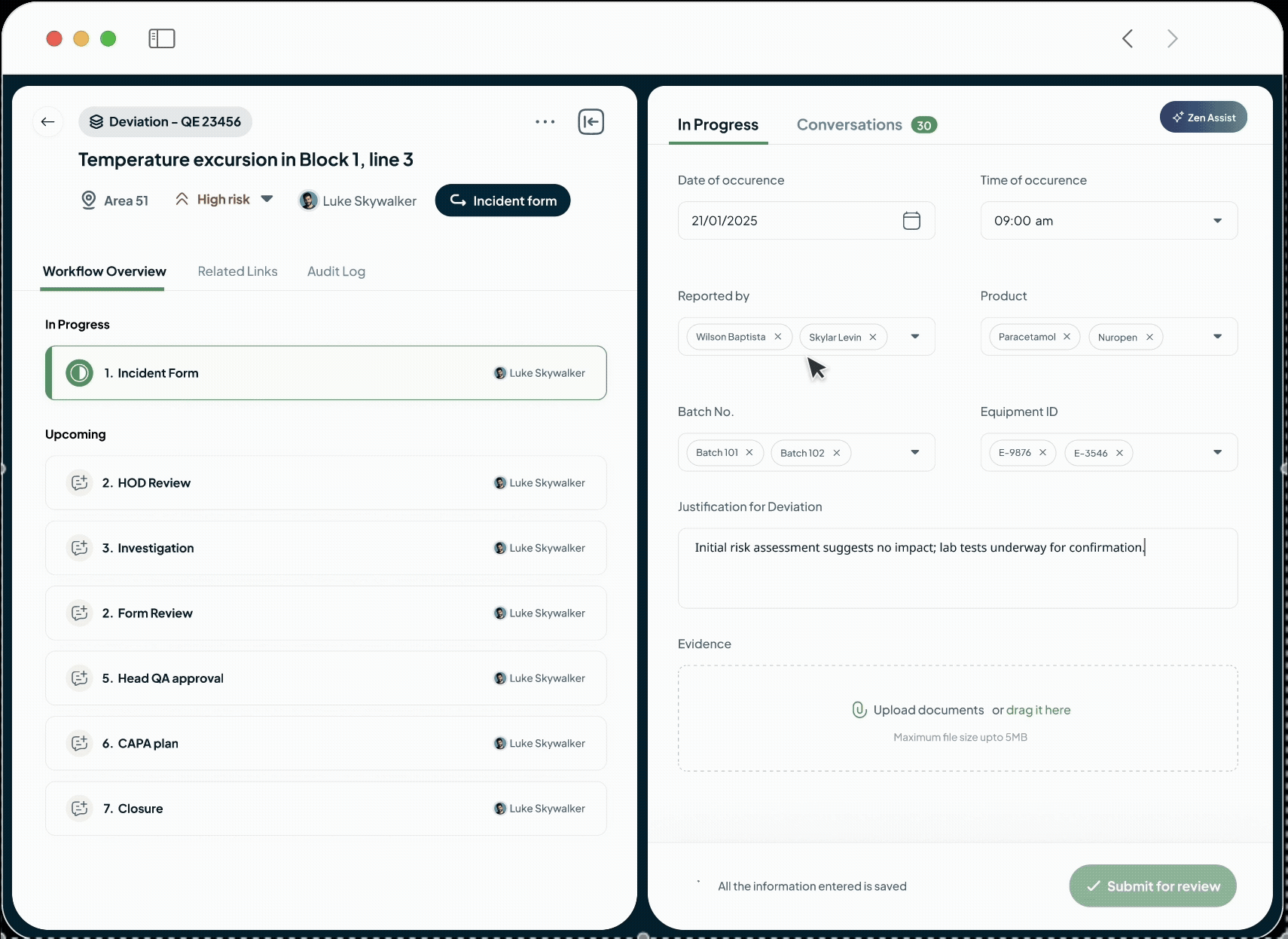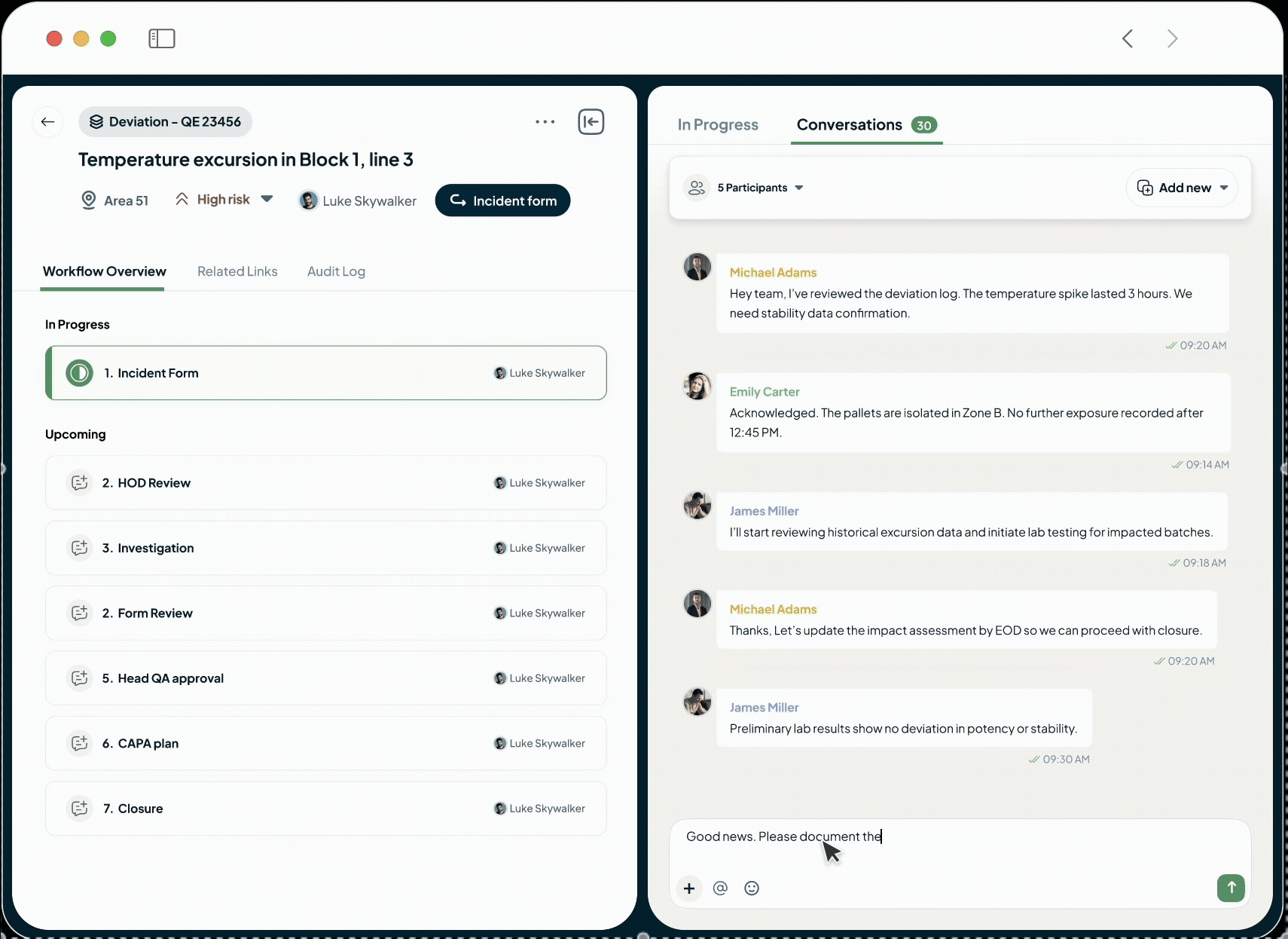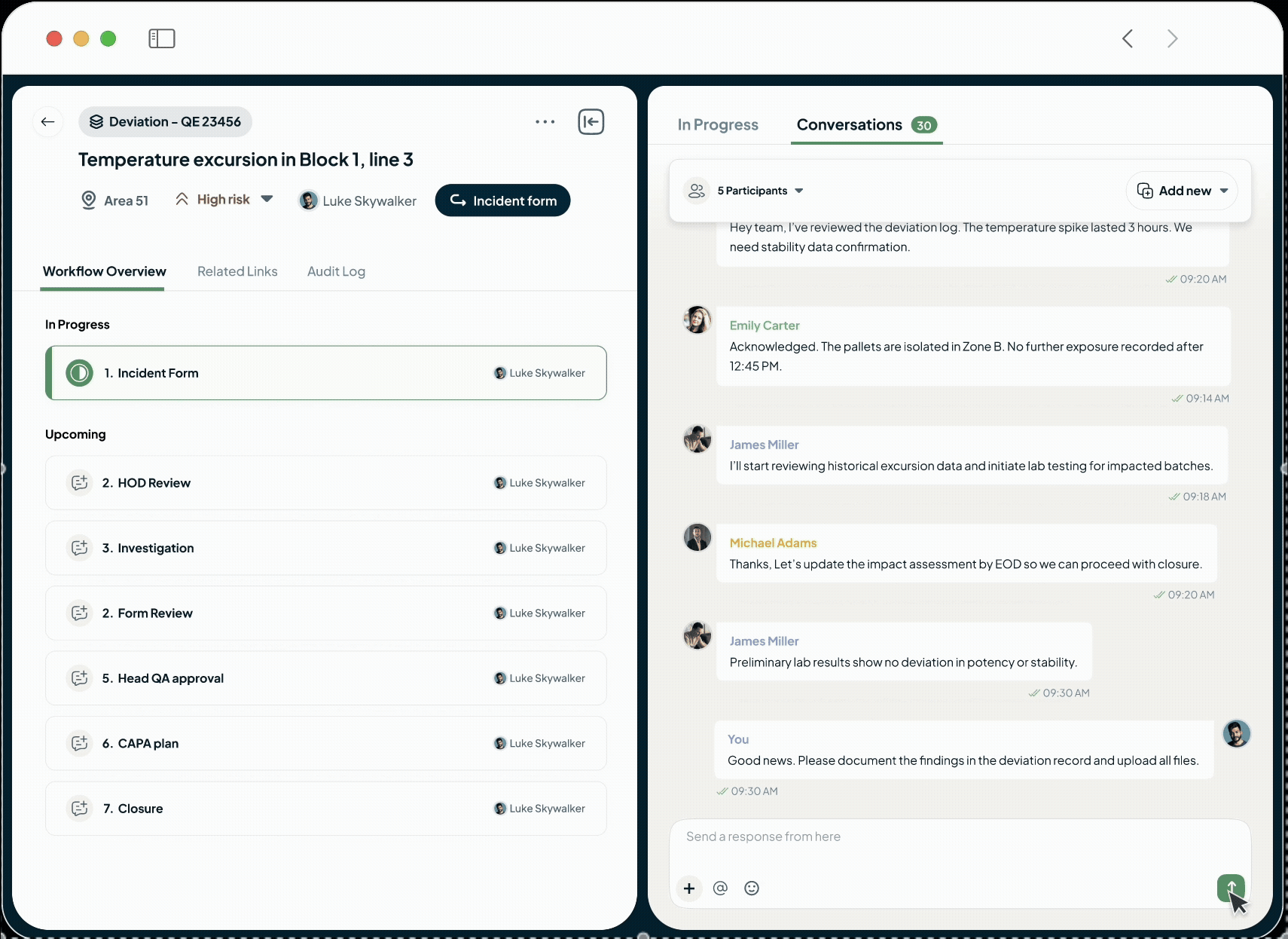
The intelligent, unified Quality Management System is designed to reduce compliance risks, support regulatory requirements (GxP, ISO, FDA 21 CFR Part 11) and enable data-driven decision-making.
Accelerate Quality Cycles
Accelerate change management by automatically identifying impacted documents, equipment, validation protocols, training programs, action items, and risks, which are auto-suggested based on historical data
Be Audit-Ready, Always
Embeds GxP and 21 CFR Part 11 compliance into every action, providing immutable, on-demand audit trails. Drastically reduce audit preparation time from weeks to hours and minimise the risk of regulatory observations
Gain Predictive Insights
Our AI-driven QMS unifies information from suppliers, complaints, and deviations to reveal hidden trends and predict risks, empowering you to prevent quality events before they occur.
A Comprehensive Suite of Features Delivered Through an Intuitive and Accessible Interface
Customizable Processes
Flexible Quality Workflows for Bespoke Process
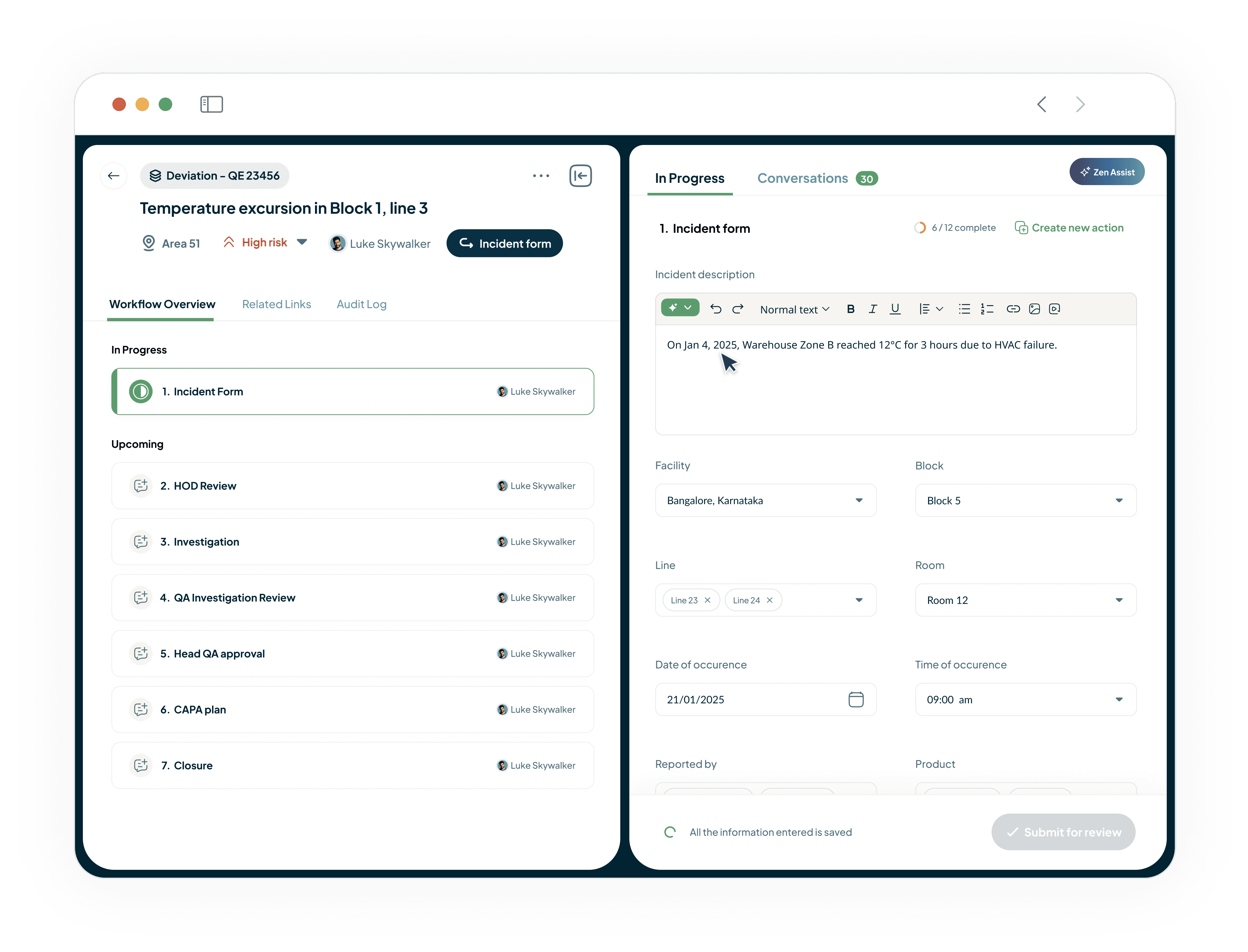
Adapt the QMS to Your Exact Processes
Our flexible QMS workflows adapt to your unique needs.
Streamline Non-conformances, Quality Events, Investigations, CAPAs, Change Controls, and Audit Management.
Configure process interlocks and ensure events are handled in a compliant way
Easy Collaboration
Collaboration-First Approach for Visibility and Accountability
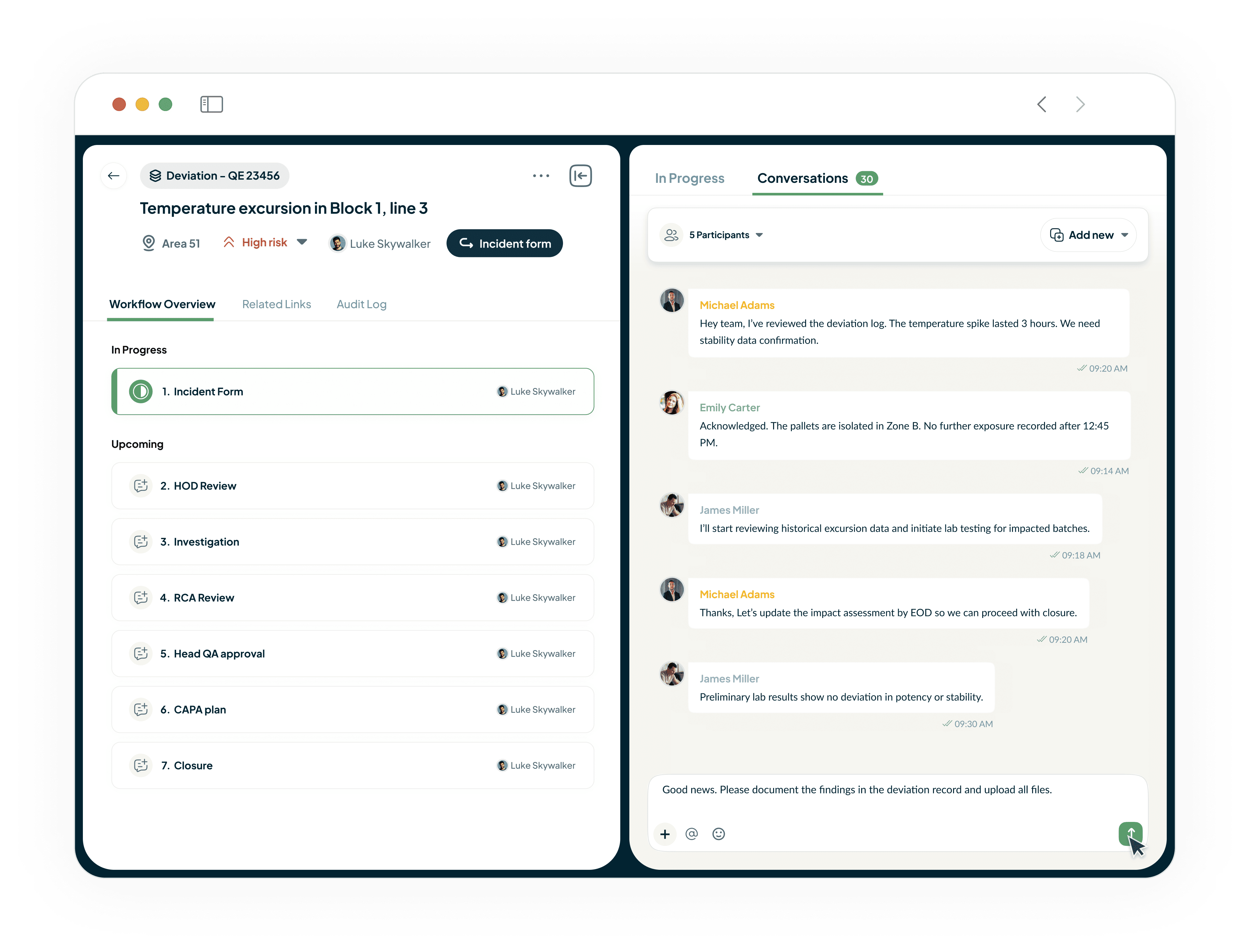
Unify Teams and Drive Accountability
Our platform eliminates information silos with built-in conversations.
Acts as your single source of truth.
Drive accountability with clear task assignments and deadlines.
21 CFR Part 11 Compliant Signatures
Audit Trails and E-Signatures for GxP Compliance
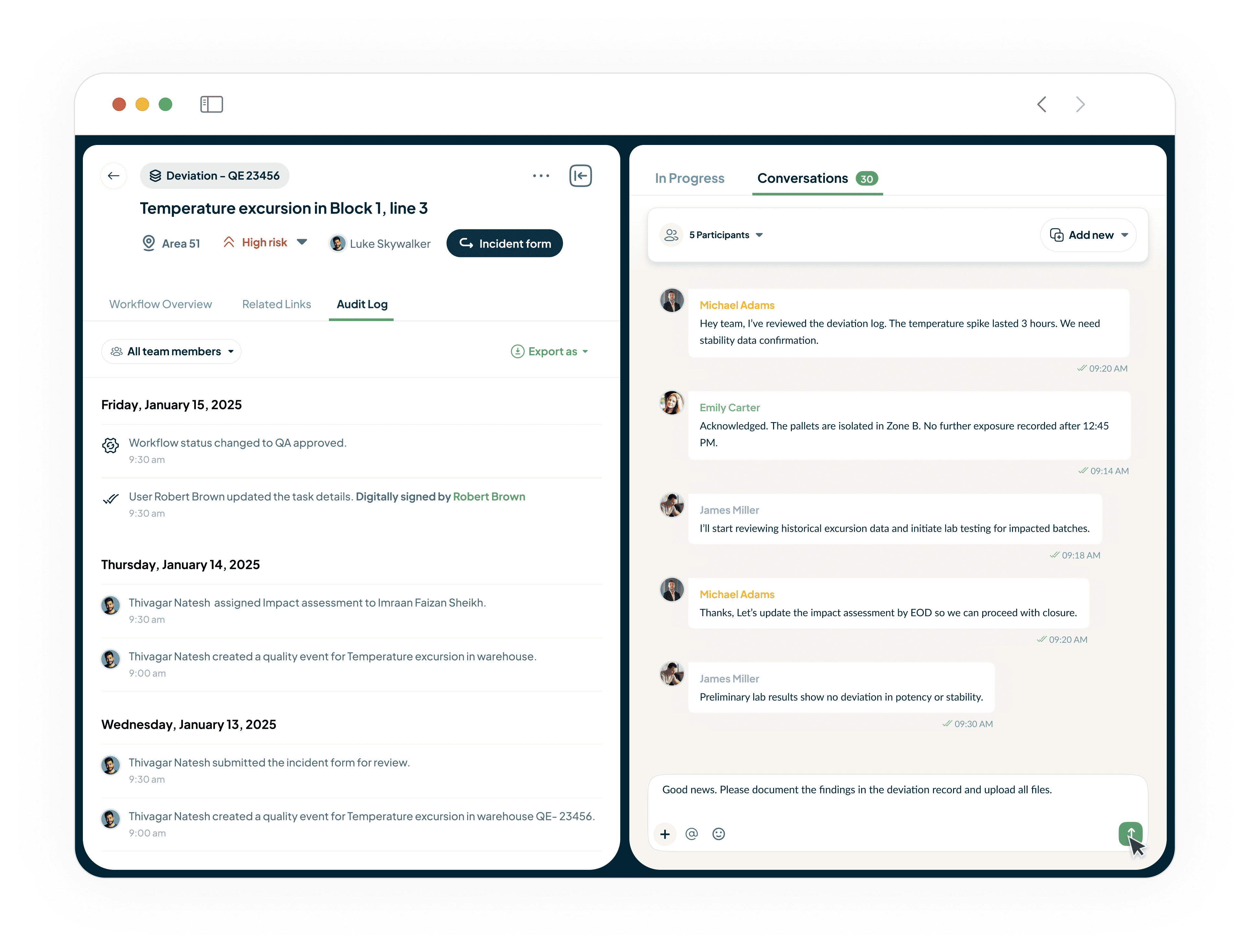
Ensure Compliance and Accelerate Approvals
Our system provides a 21 CFR Part 11-compliant audit trail for every action.
Secure e-signatures eliminate manual processes.
Ensures timely execution.
Smart Analytics
Rich Reporting and Custom Dashboards
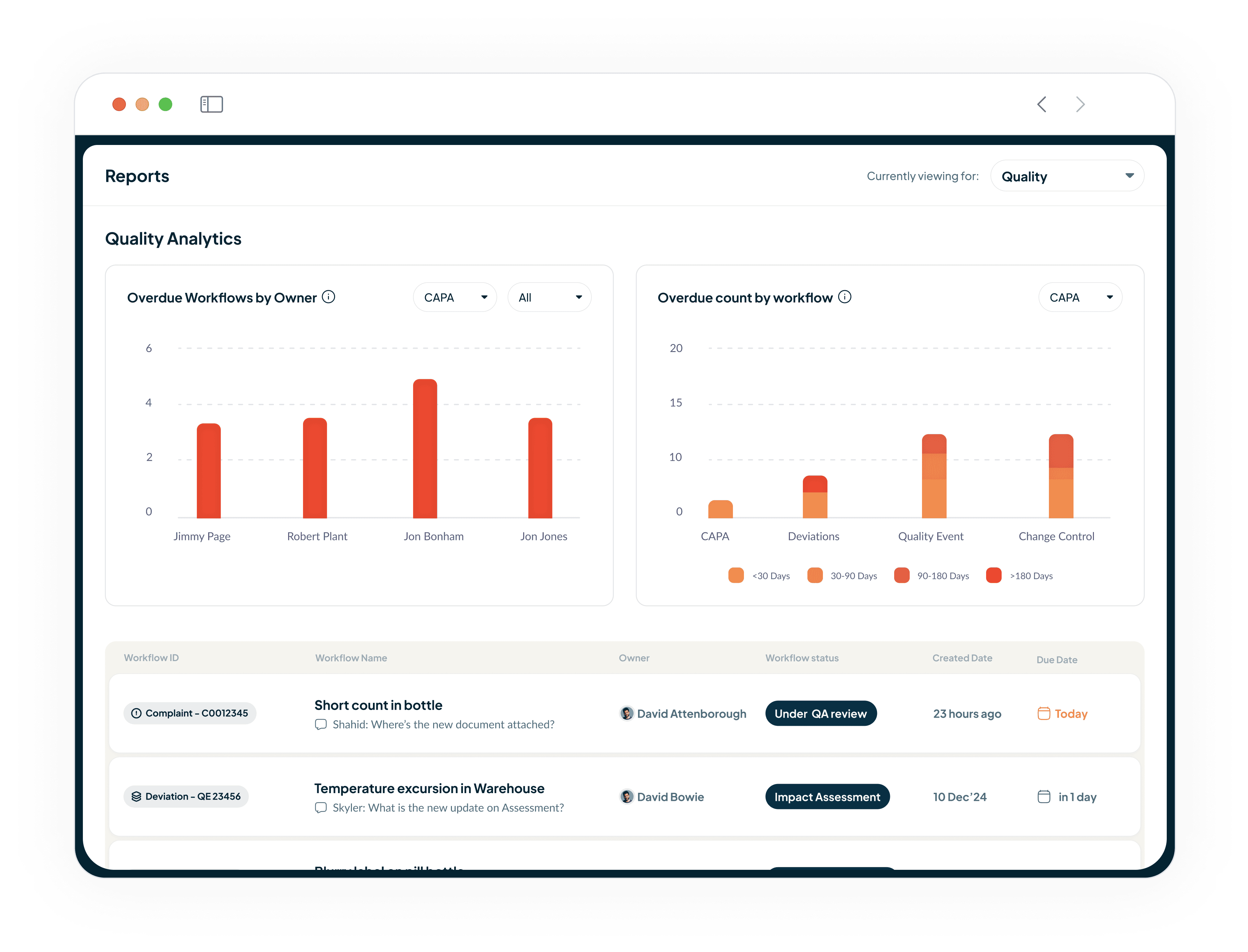
Gain Deep Insights into Quality Performance
Our powerful QMS reporting and analytics dashboards provide deep insights.
Helps you analyse frequent issues and overdue events.
Uncover operational bottlenecks that were previously invisible.
Controlled Access
Fine-Grained Access Control Privileges
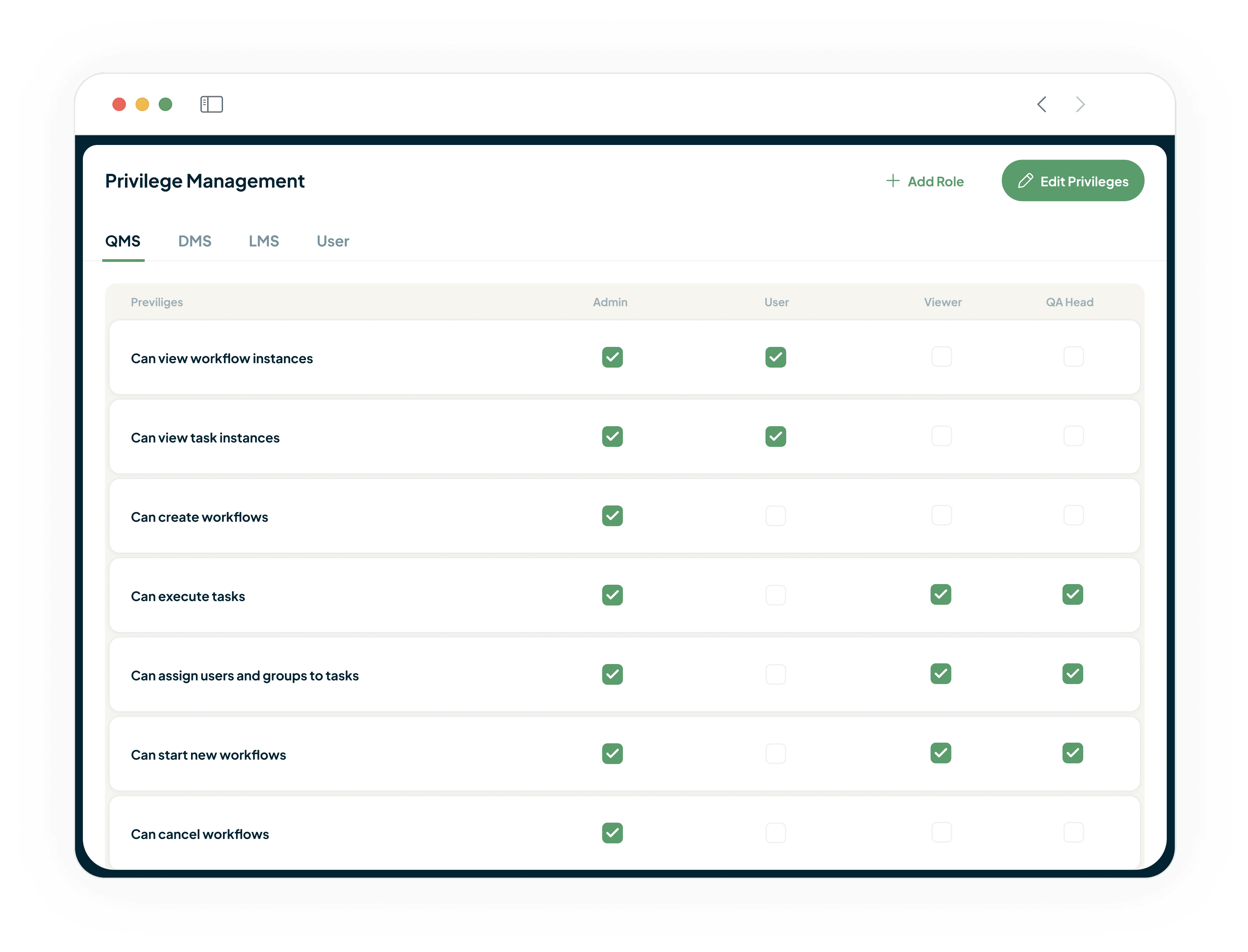
Protect Sensitive Data and Ensure Integrity
Our fine-grained access control privileges ensure only authorised personnel can access sensitive information.
Maintains data integrity and regulatory compliance







