AI-Powered
Manufacturing Execution System
Accelerate batch release from weeks to days with "review by exception." Our MES replaces paper with dynamic Electronic Batch Records (eBRs) that guide operators, prevent manual errors, and generate a complete, GxP-compliant record for every run.
The intelligent, no-code manufacturing execution system is designed to eliminate paper-based processes, ensure FDA 21 CFR Part 11 compliance, and accelerate batch release.
Reduce Process Deviations
Our platform enforces your process with digital interlocks and guided workflows, actively preventing errors before they occur and guaranteeing consistent, high-quality batch execution.
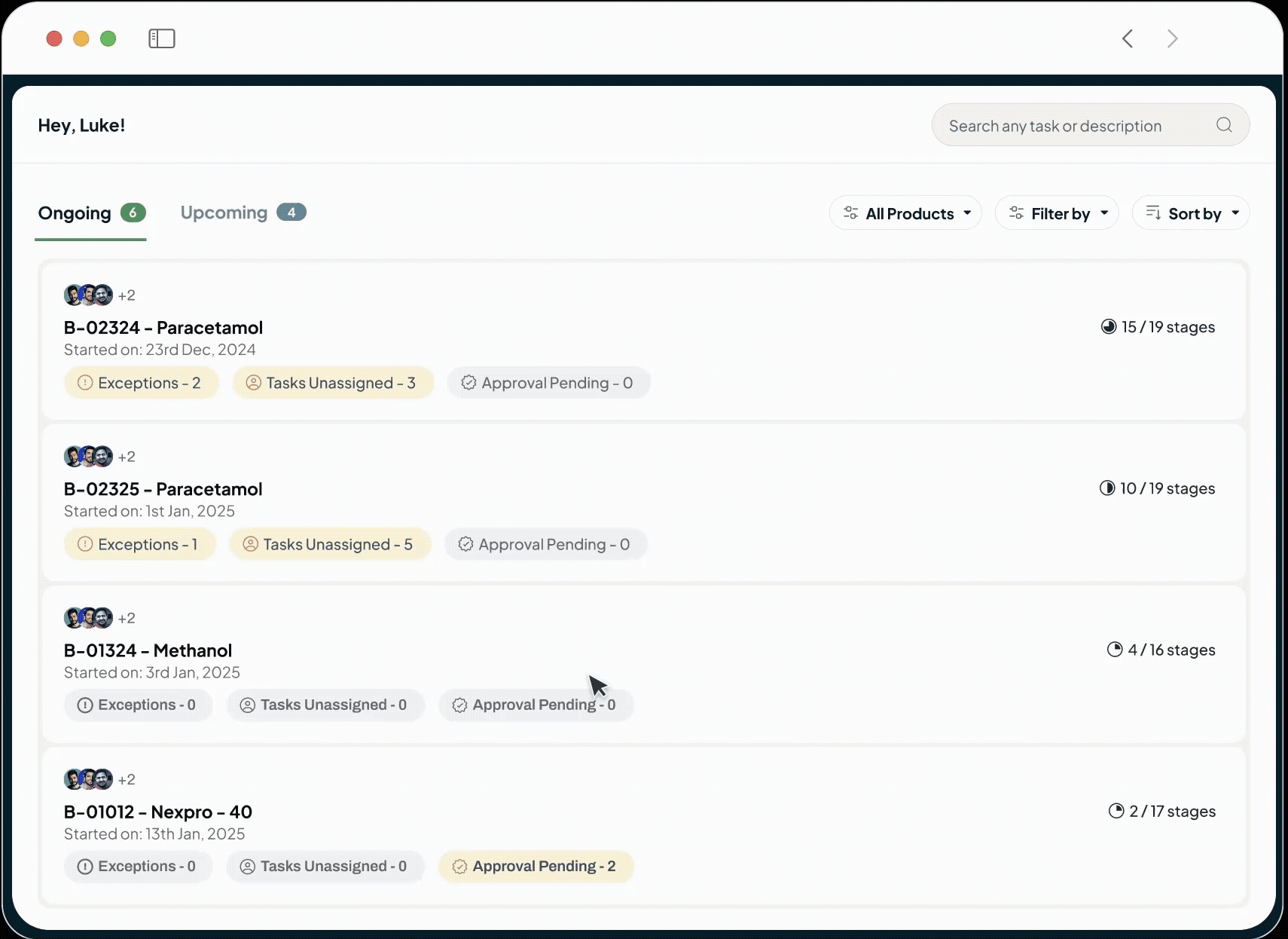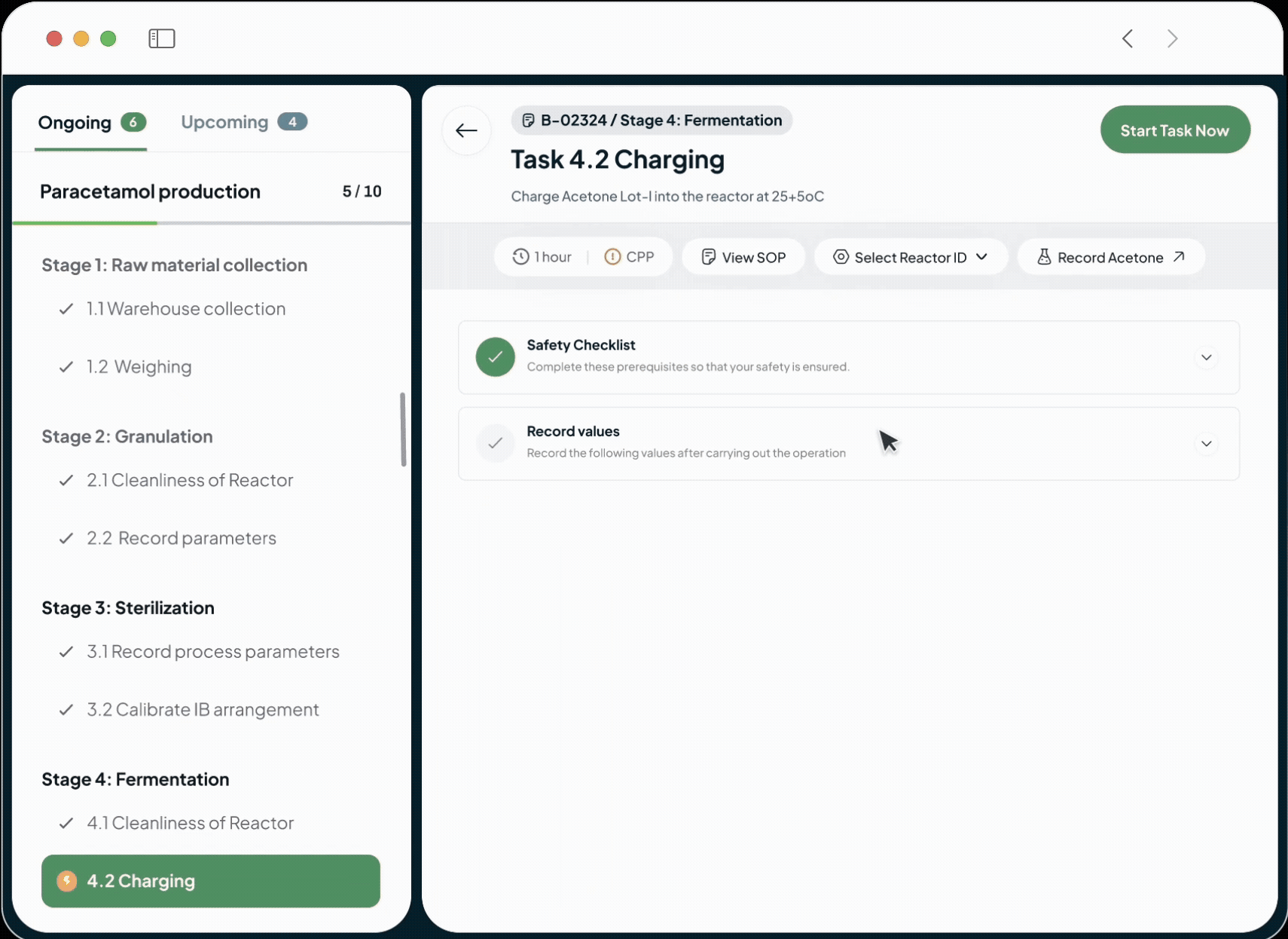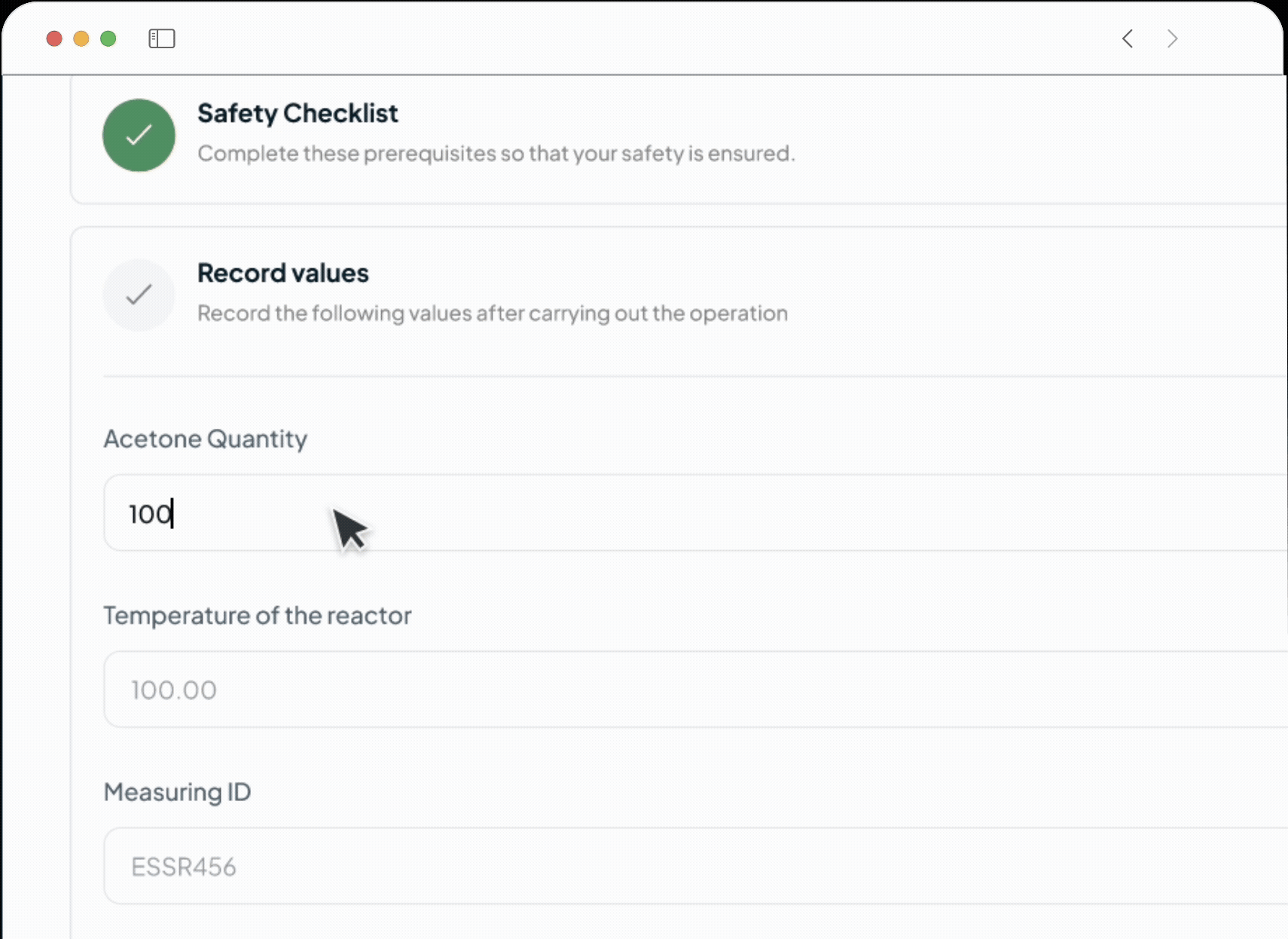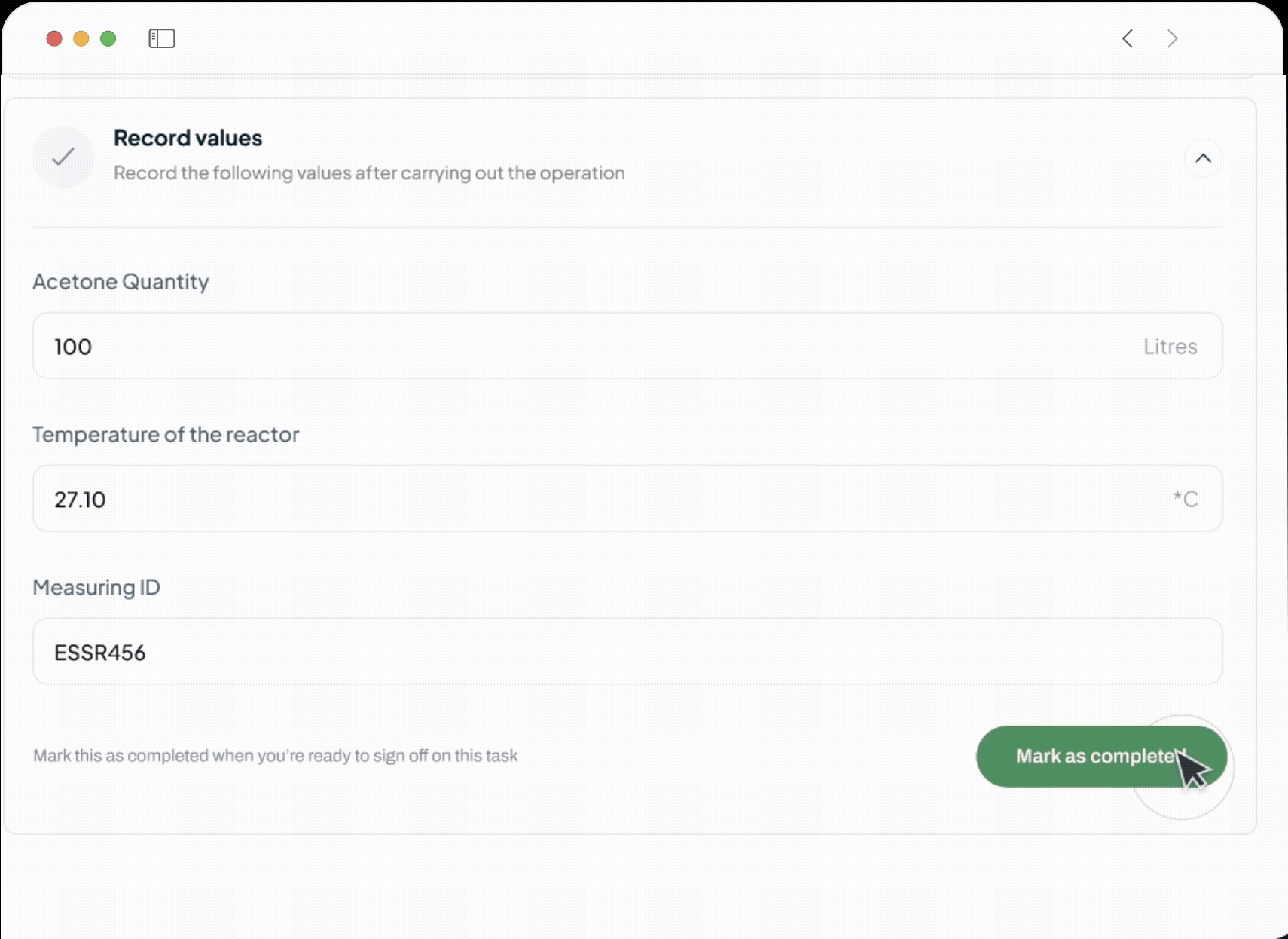
Expedite Batch Release
Our system tracks all exceptions as they occur and presents a complete, data-perfect electronic batch record to QA, streamlining review cycles and speeding compliance.
Minimise Production Delays
Our platform provides a unified, real-time view of your resources, automatically verifying that all materials, equipment, and personnel are ready and qualified before a batch even begins.
A Comprehensive Suite of Features Delivered Through an Intuitive and Accessible Interface
Electronic Batch Record
Electronic Batch Record to Reduce Deviations
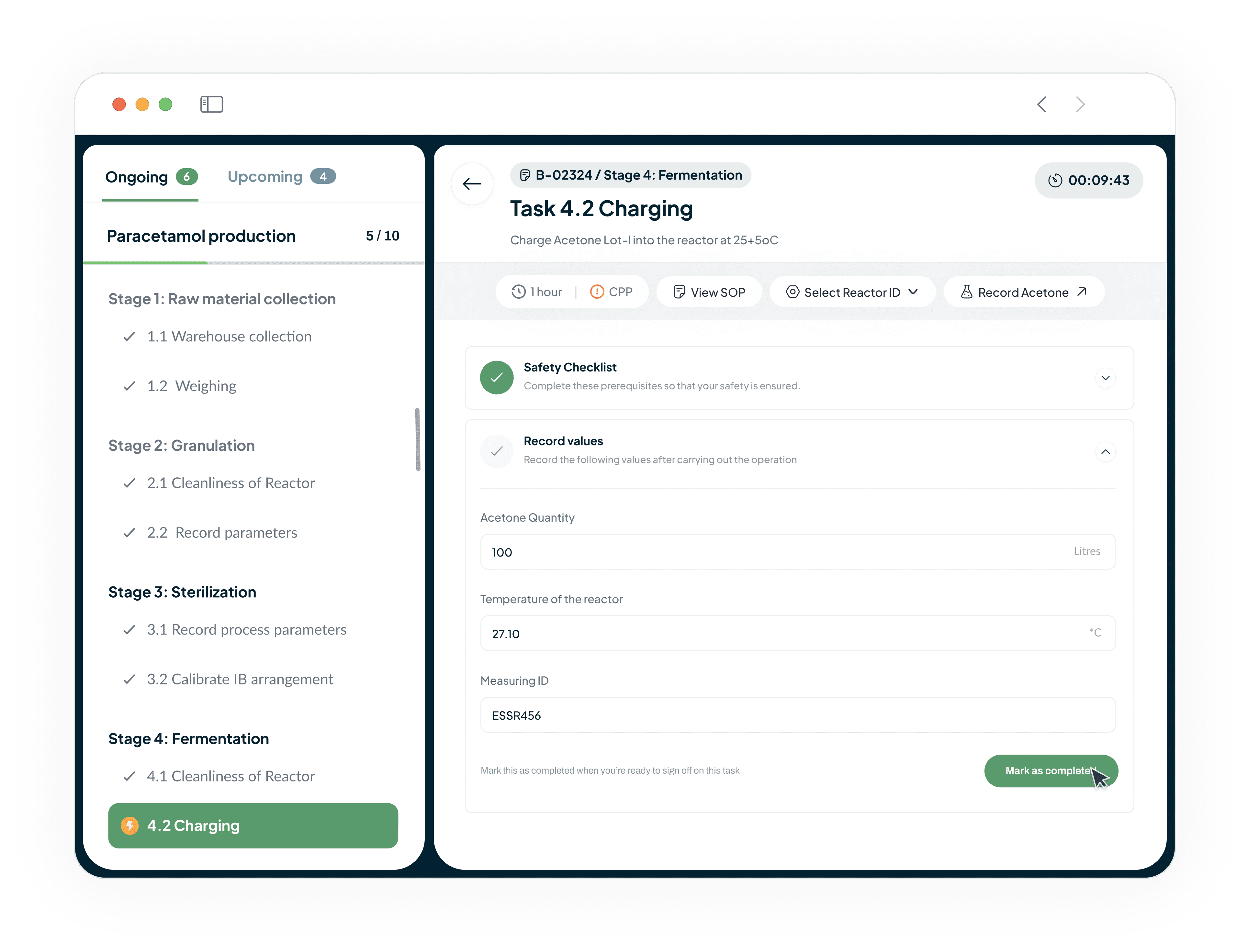
Guide Operations and Enforce SOP Adherence
Our system guides operators through each step of a process with intuitive instructions.
Automated checks and process interlocks are seamlessly integrated
Enforce strict adherence to Standard Operating Procedures (SOPs) and ensure right-first-time execution
Prevents manual errors and costly batch failures.
Digital Twin
Track All Shop Floor Activity
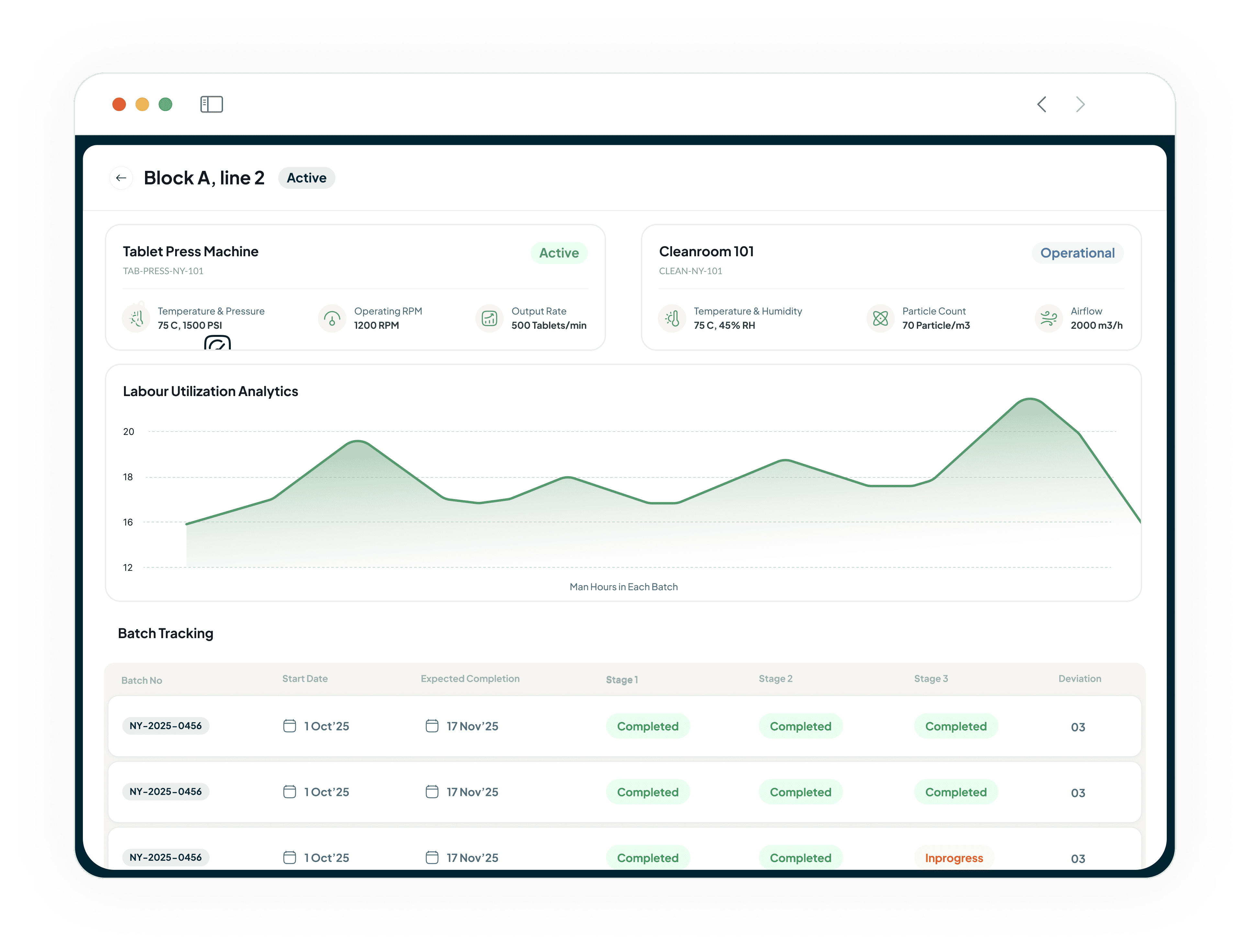
Gain Real-Time Visibility and Optimise Management
Gain real-time visibility into your facility’s operations with shop floor software.
Access comprehensive insights into equipment status, batch progress, and process efficiency.
Optimise shop floor resource allocation.
Auto Capture
Capture Weighing Records Automatically
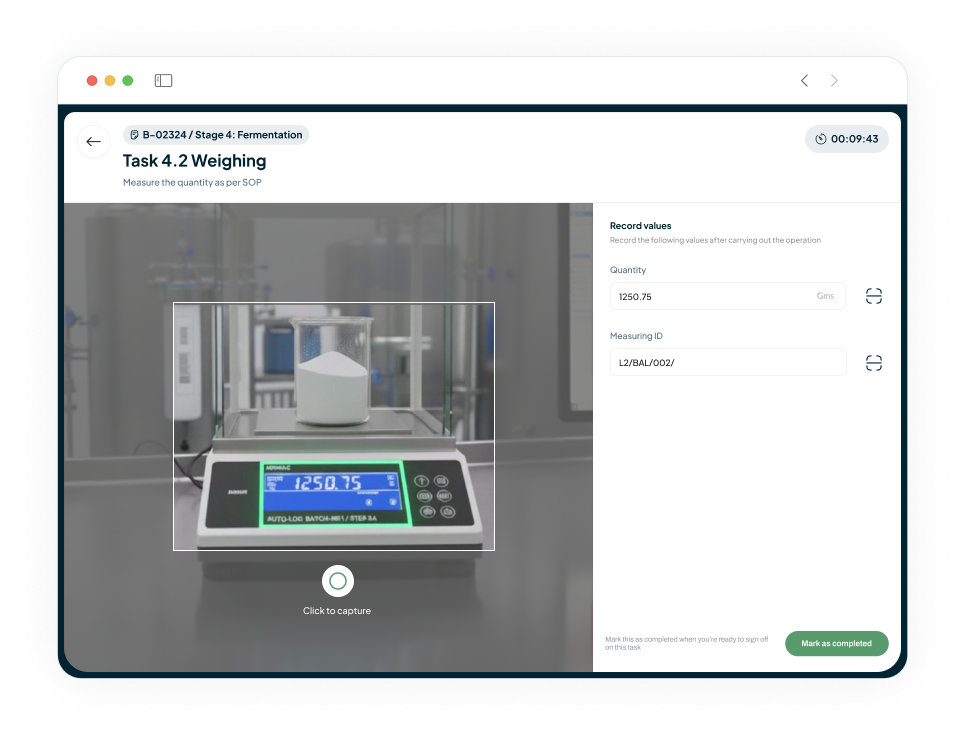
Eliminate Manual Errors in Weighing and Dispensing
Our AI system automates the logging of precise weighing.
Enforces strict tolerance limits to prevent deviations.
Eliminates manual errors and ensures quality.
Audit Trails
GDP and 21 CFR Part 11 Compliant Digital Records
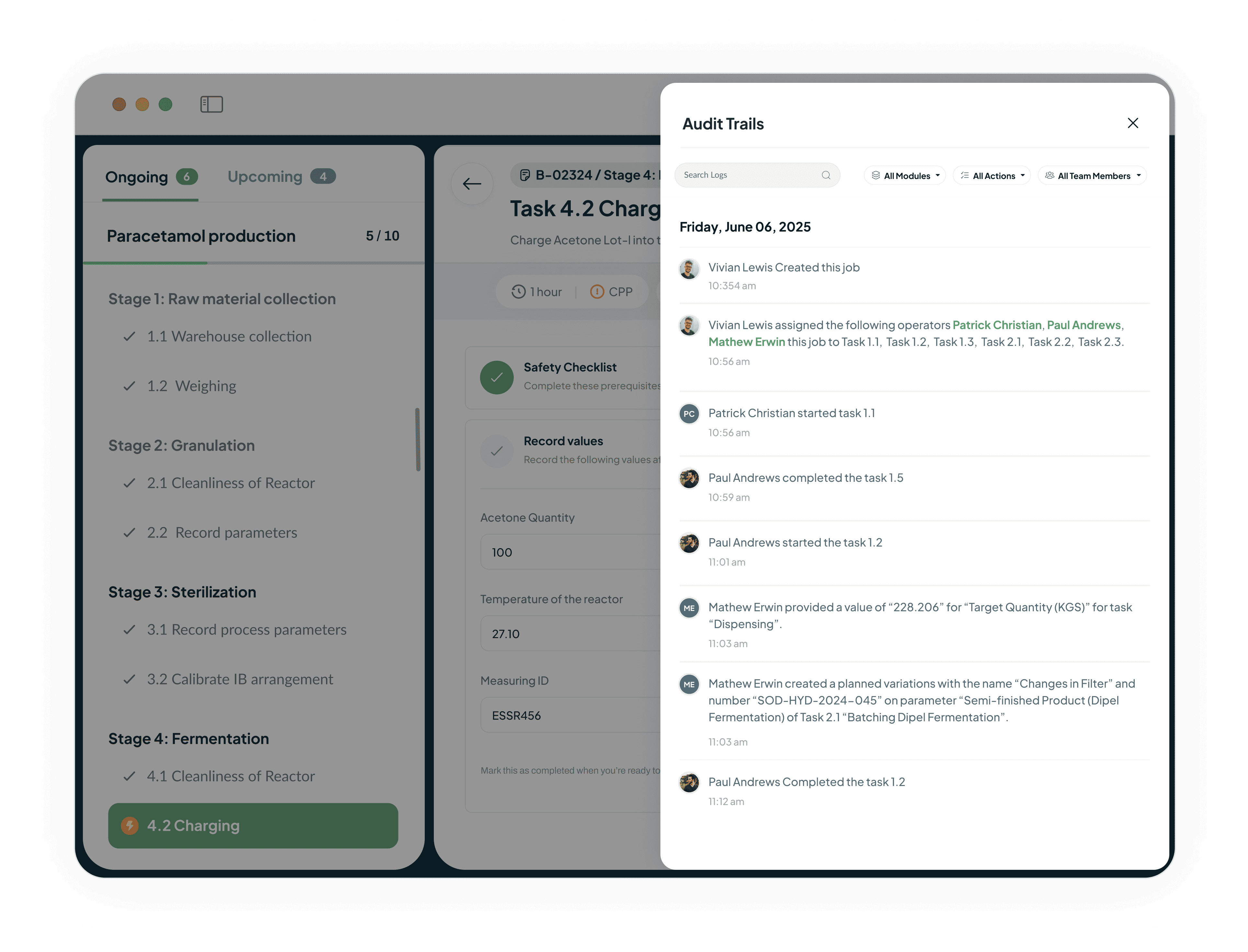
Ensure Audit-Ready Data Integrity
The system generates tamper-proof audit trails for every action.
Provides a complete and verifiable history of all shop floor activities.
This robust record-keeping capability facilitates instant regulatory reporting.







