AI-Powered
Learning Management System
Move beyond spreadsheets for managing your GxP training and compliance. When an SOP is updated in your DMS, our LMS automatically triggers and tracks the required training, closing compliance gaps before they happen
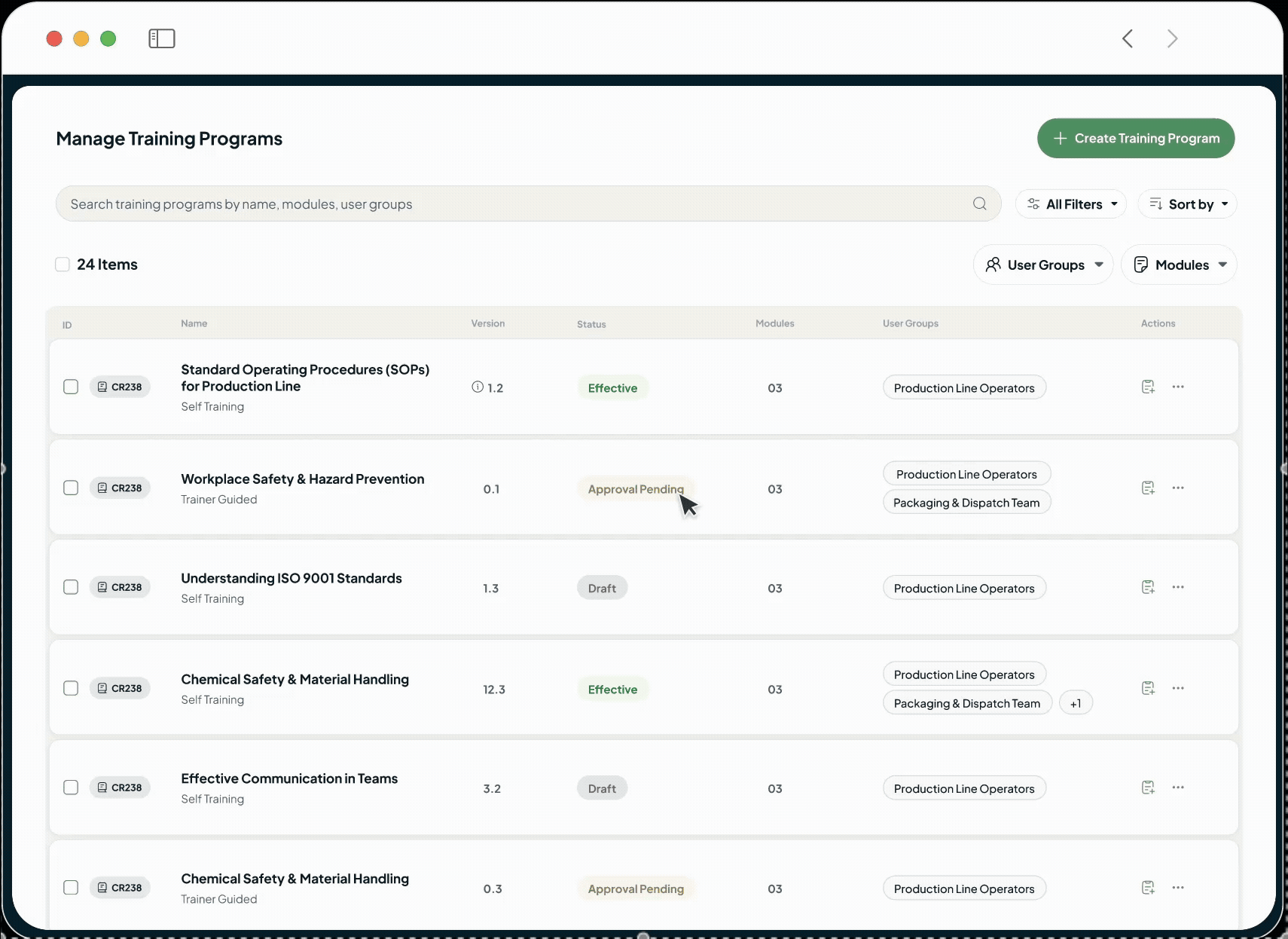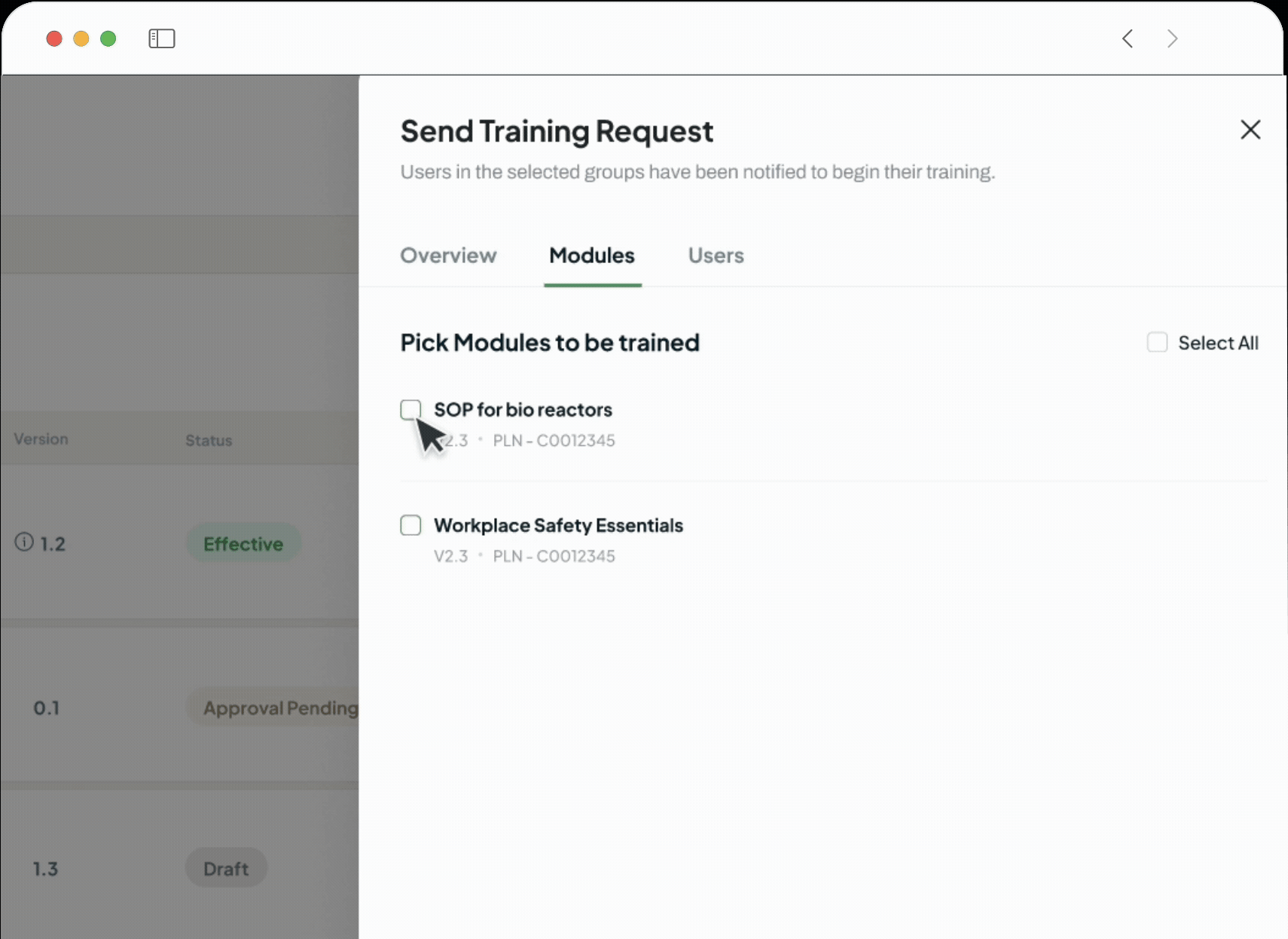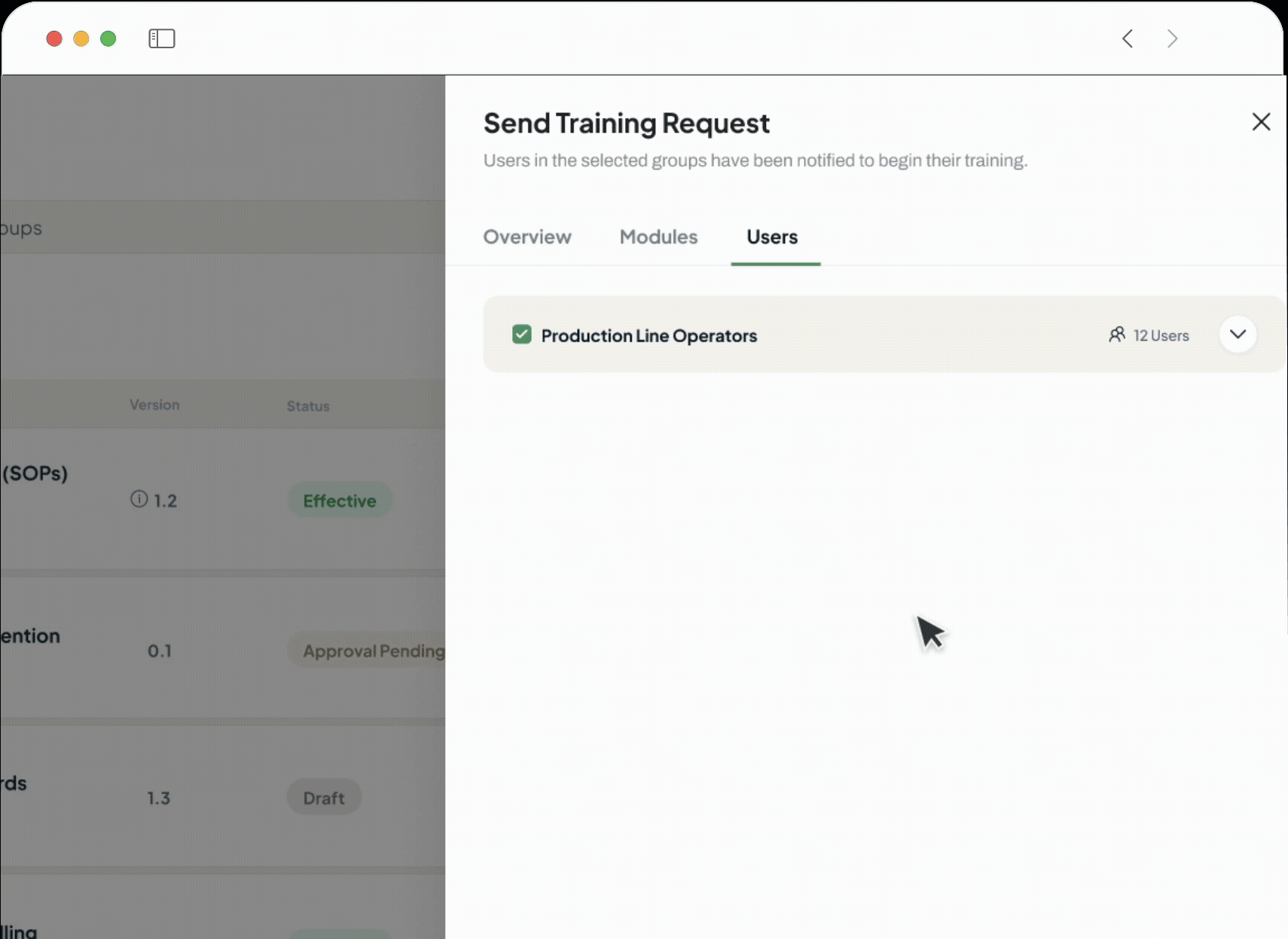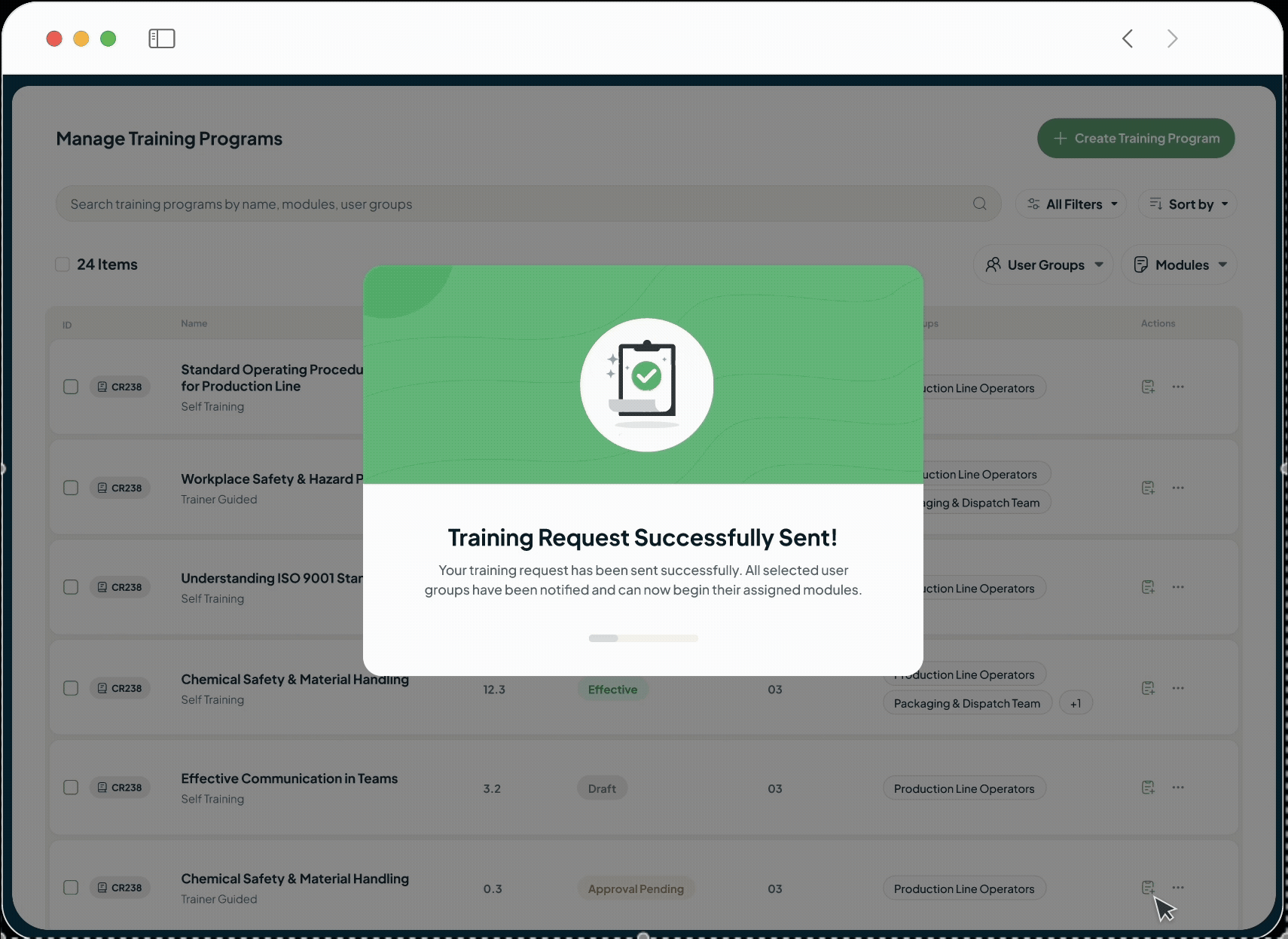
The intelligent GxP-compliant Learning Management System is designed to close critical compliance gaps and replace administrative burden with verifiable, audit-ready training records, which are perfect for pharmaceutical, biotech, and life sciences companies.
Reduce Compliance Risks
Eliminate compliance risks by linking training directly to the document control system. SOPs only become effective after all required personnel are verifiably trained and assessed, ensuring FDA 21 CFR training compliance.
Automated Tracking
Automate role-based assignment, reminder notifications and progress tracking with our AI LMS, free your training managers to focus on developing effective learning content and programs.
Verifiable, Audit-Proof Learning
Enforce true learning with integrated assessments, qualify instructors for guided sessions, and capture every action with 21 CFR Part 11 compliant e-signatures and a complete, immutable audit trail.
A Comprehensive Suite of Features Delivered Through an Intuitive and Accessible Interface
Comprehensive Training
Standardise & Automate Role-Based Learning
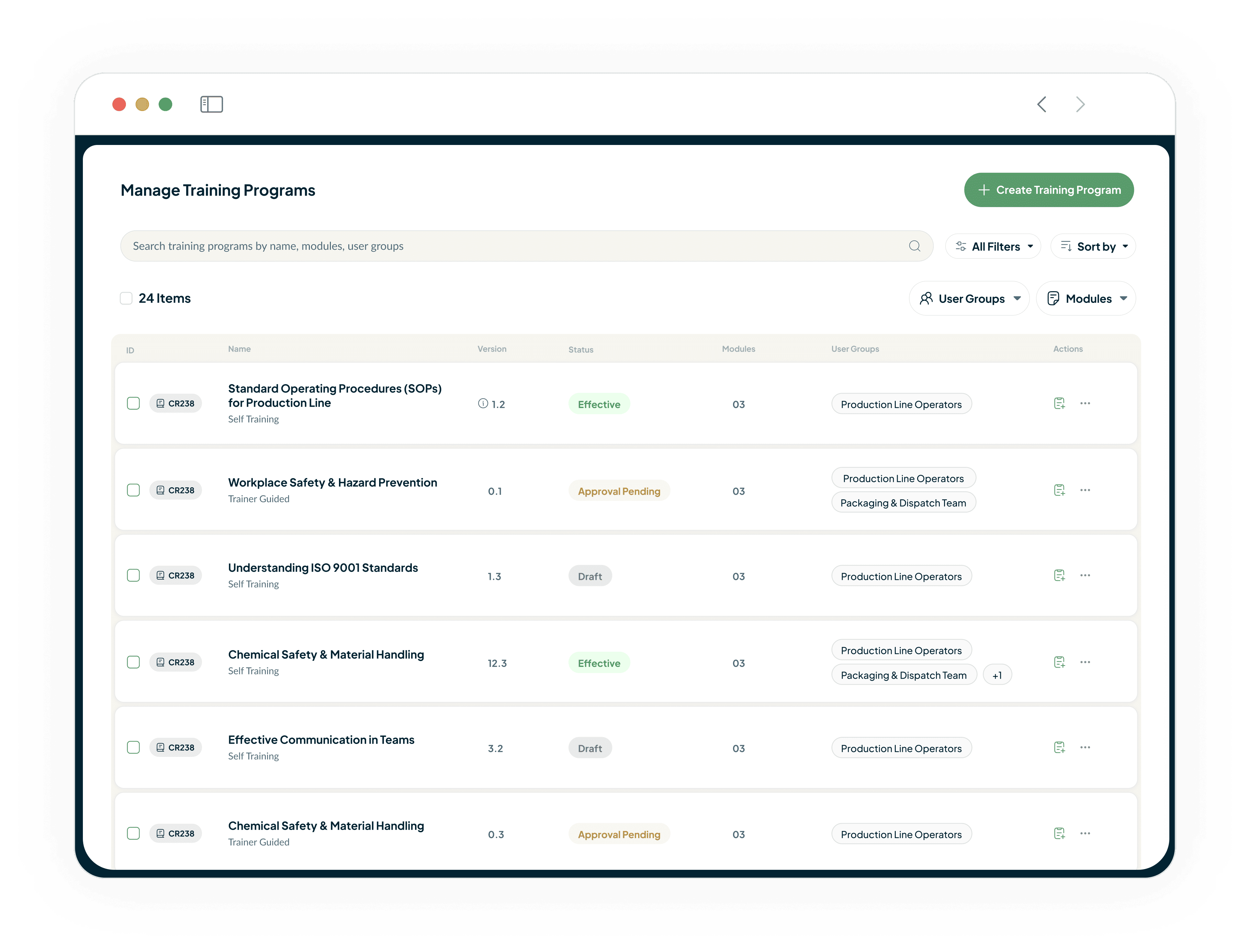
Ensure Consistent GxP Role Training
Eliminate the risk of inconsistent, manual training assignments
Define version-controlled and complete training programs for every GxP role.
Ensure every team member, from the lab to the production floor, gets the exact training they need.
Be compliant and audit-ready from day one.
Personalised Learning
A Personalised Hub for Compliant Learning
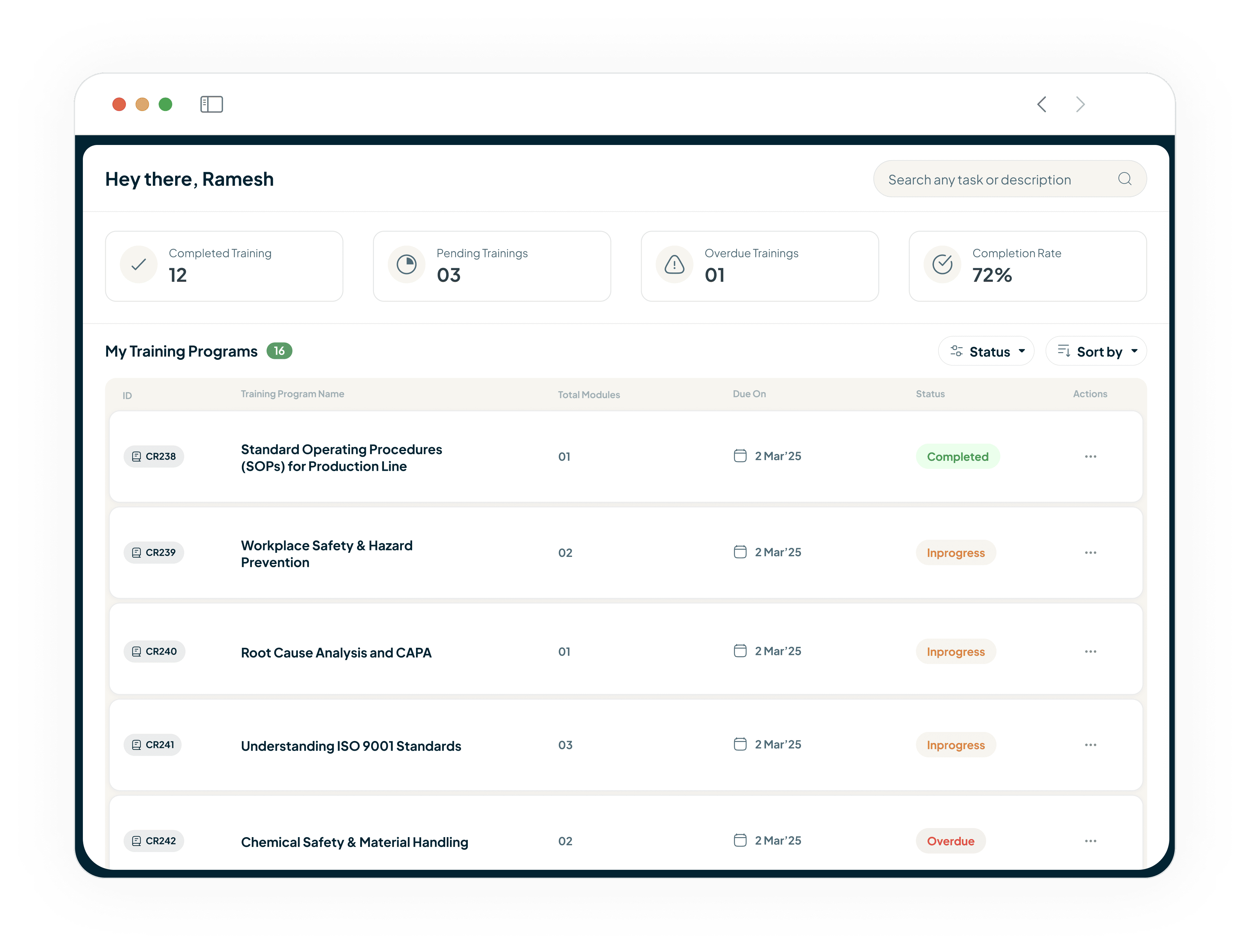
Empower Users with a 21 CFR Part 11 Compliant Hub
Provide your team with a single dashboard
Users can view pending tasks, complete readings, and take assessments.
Every action is captured in a fully compliant, 21 CFR Part 11 auditable environment.
Flexible Training
Flexible Training Mandates
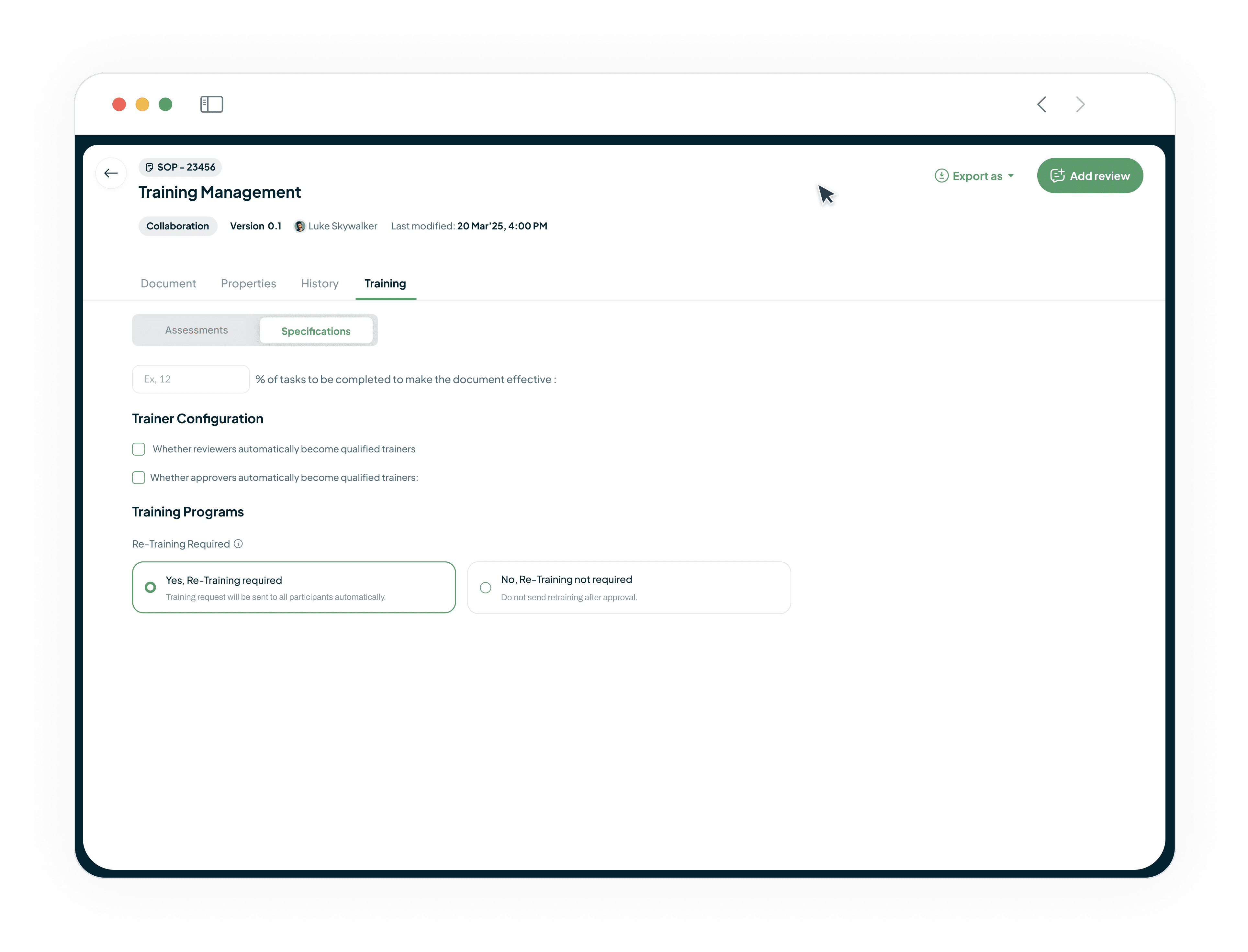
Customizable Training for Any Document
Configure multiple assessments and required training rules for any document.
Set rules for requiring retraining on major updates.
Set team completion percentages for a document to become effective.
Audit-Ready Training
Instant, Audit-Ready Training Records
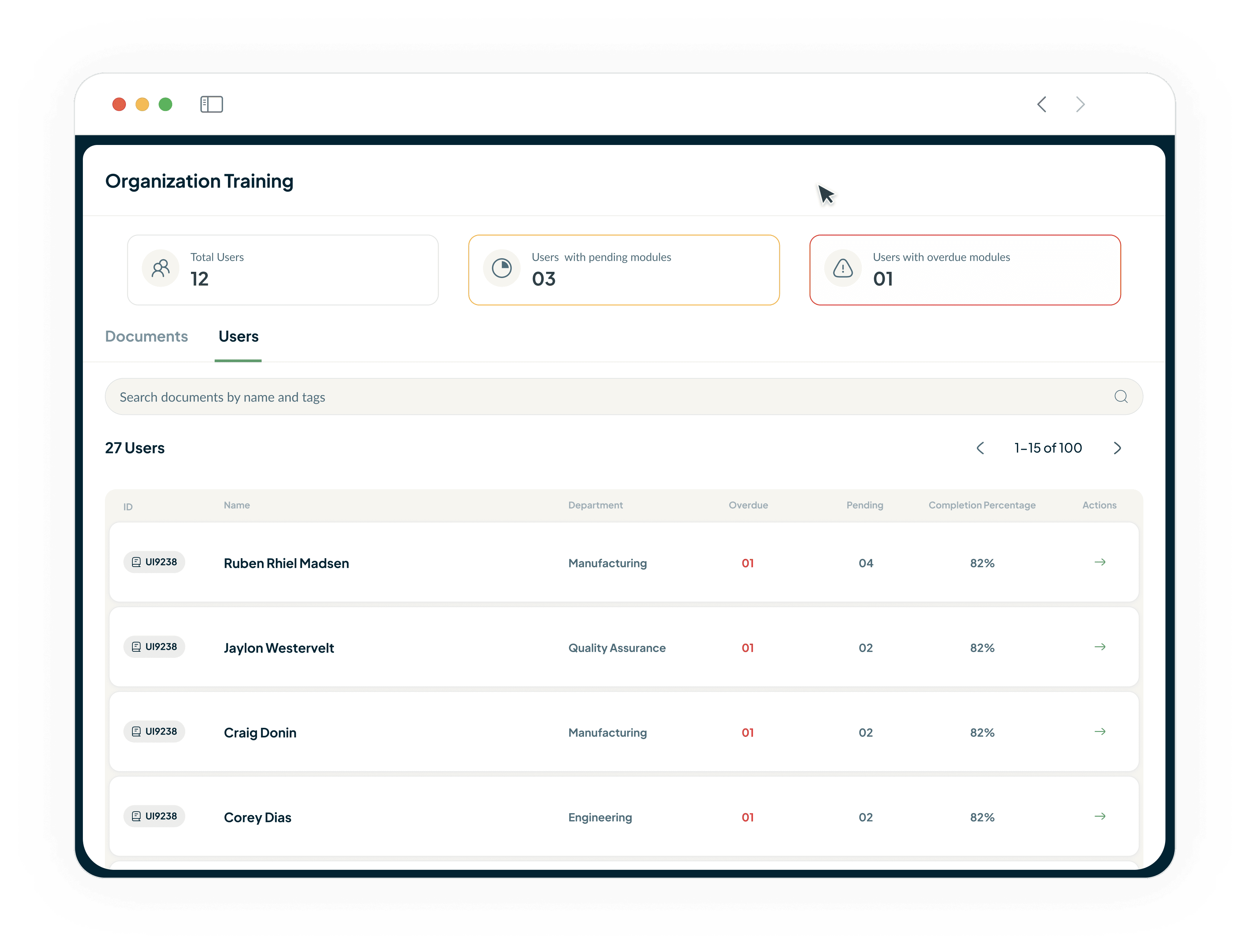
Be Inspection-Ready at All Times
Access a complete, uneditable history of every learning activity.
All records are in a centralised database.
Export comprehensive reports for auditors in seconds.
AI-Driven Training
Accelerated Training Processes with AI
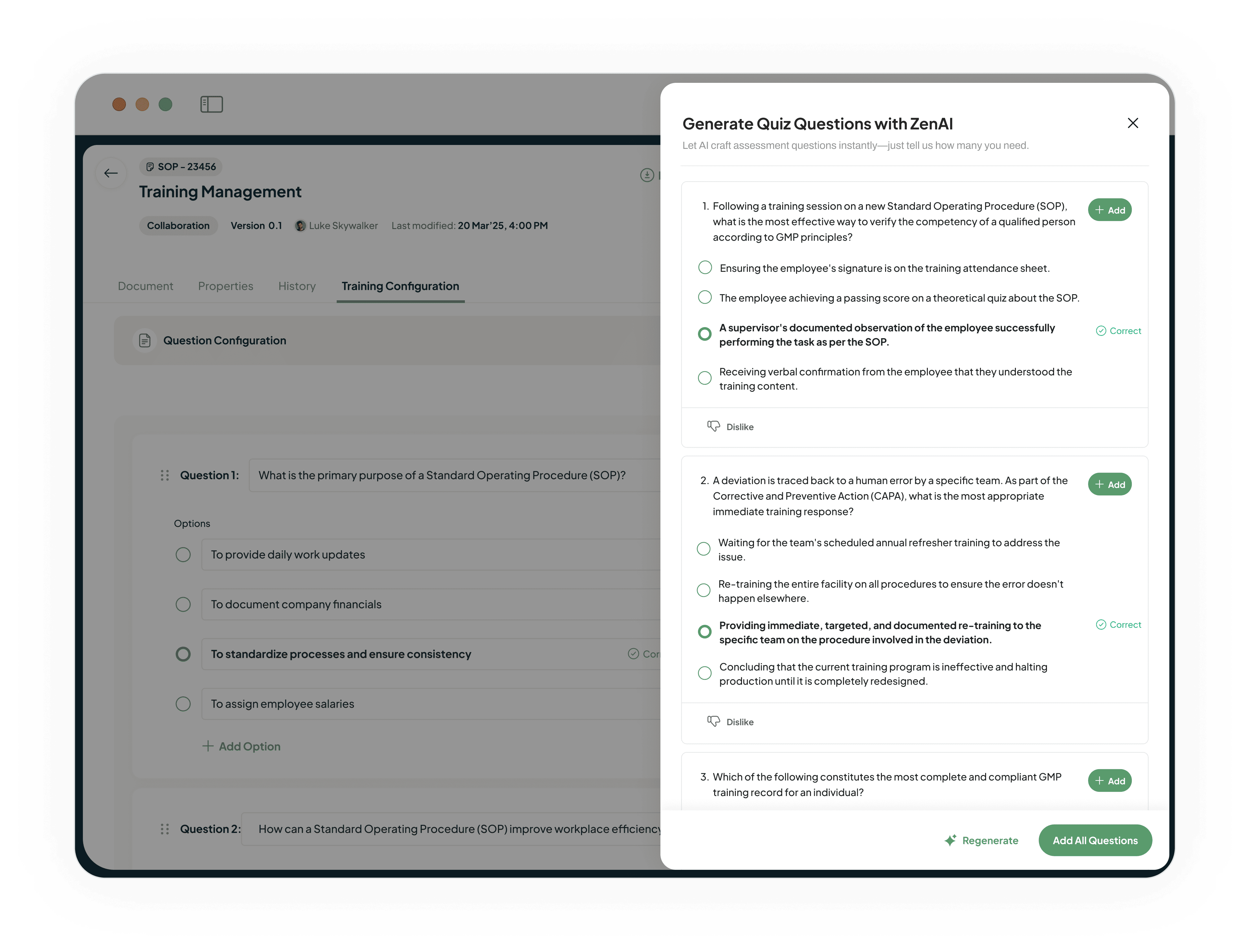
Automate Assessment Generation with AI
Our AI-driven LMS reads your documents as they're created.
Automatically generates relevant assessment questions.
Allows you to embed verifiable competency checks into your training process.







