AI-Powered
Document Management System
Get GxP-compliant control over your documents without the complexity. Easily manage the entire lifecycle, from drafting with AI assistance to secure distribution and 21 CFR Part 11 compliant approvals, all in one intuitive, centralised system.
The intelligent document management system simplifies how life sciences teams author, approve, and manage their most critical GxP documentation while ensuring regulatory compliance.
Author and Approve SOPs in Record Time
Our intuitive in-built editor with a powerful AI co-author enables your team to create clear, consistent Standard Operating Procedures from templates in minutes, all within a single, controlled document management platform.
Be Permanently Inspection-Ready
Ensure GxP compliance with immutable audit trails, validated FDA 21 CFR Part 11 e-signatures, and automated periodic review cycles. Produce any required document, including version history and approval records, instantly.
5x Faster Change Management
Our AI automatically identifies impacted documents and suggests changes instantly, allowing faster execution in a controlled, auditable environment
A Comprehensive Suite of Features Delivered Through an Intuitive and Accessible Interface
AI- Powered Tools
Industry First Built in AI Document Editor
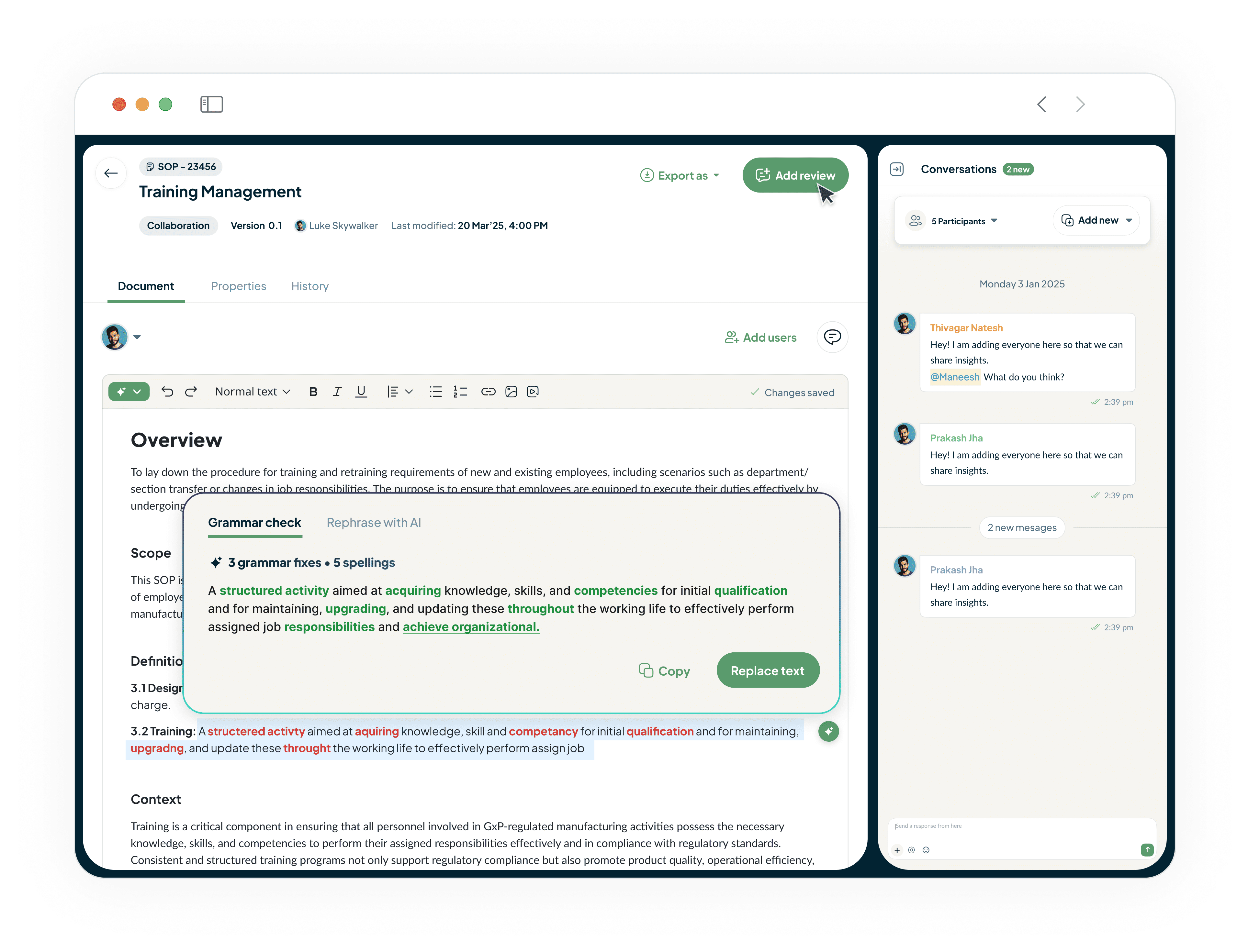
Draft Compliant Documents in Minutes, Not Weeks
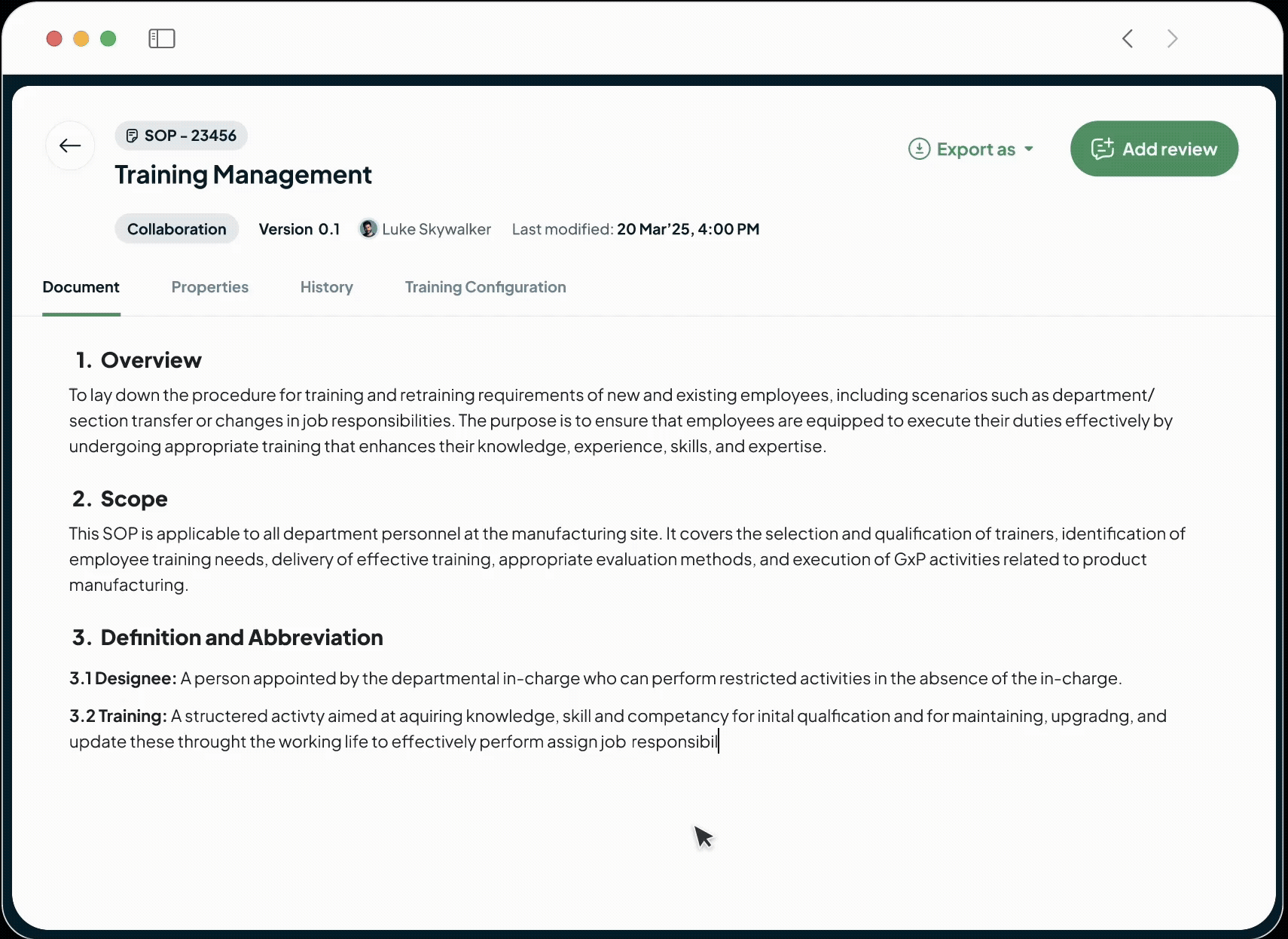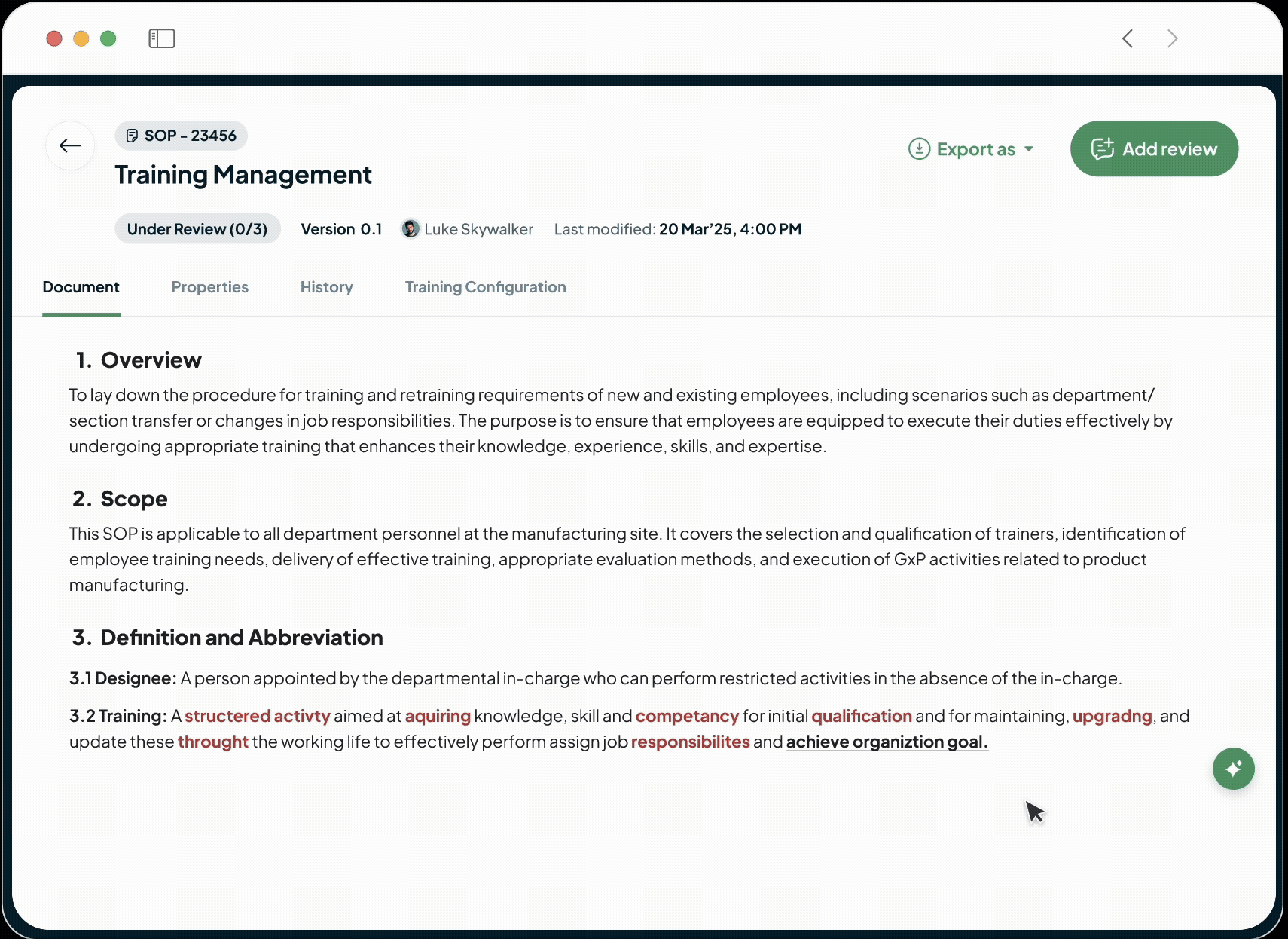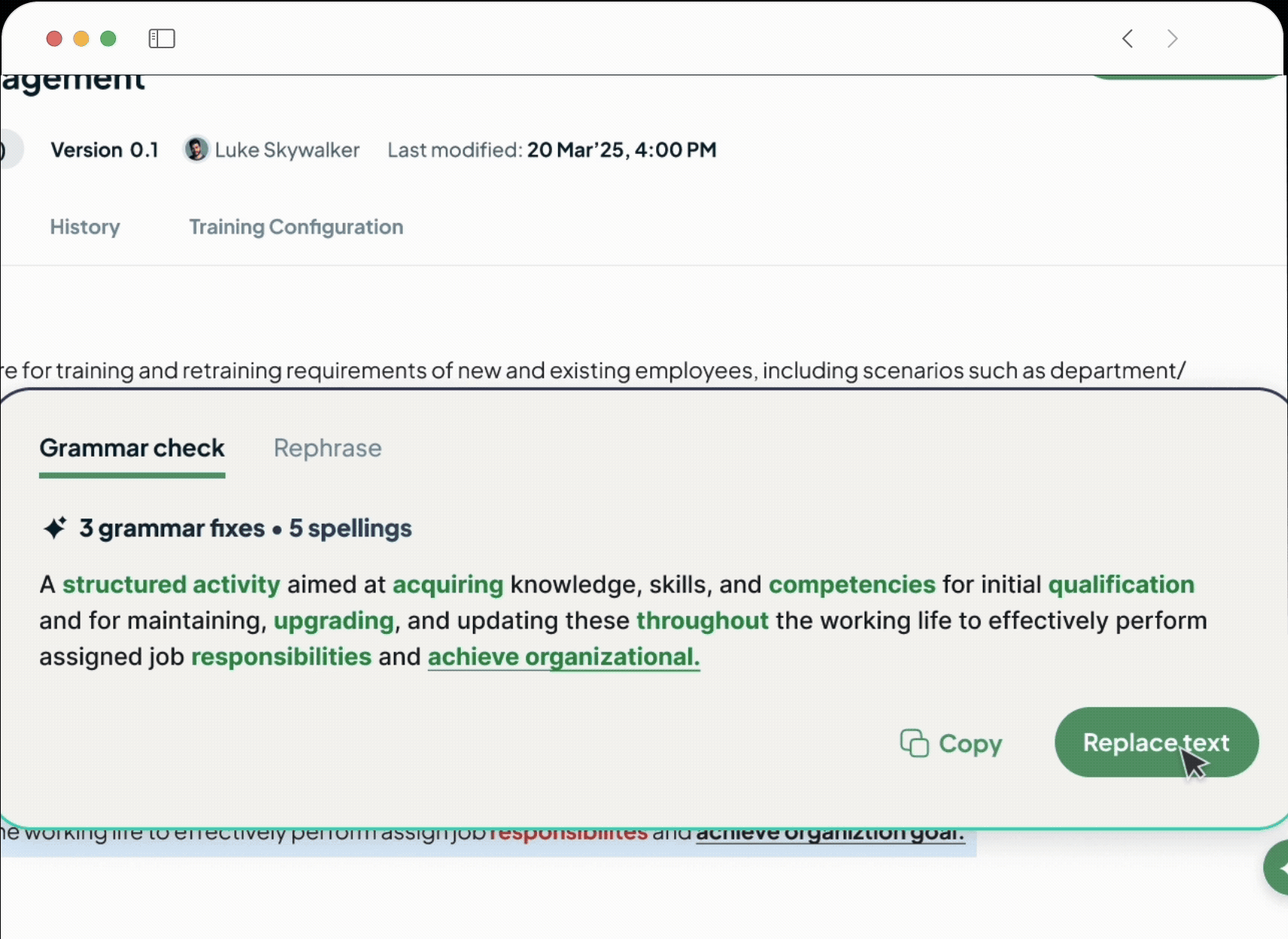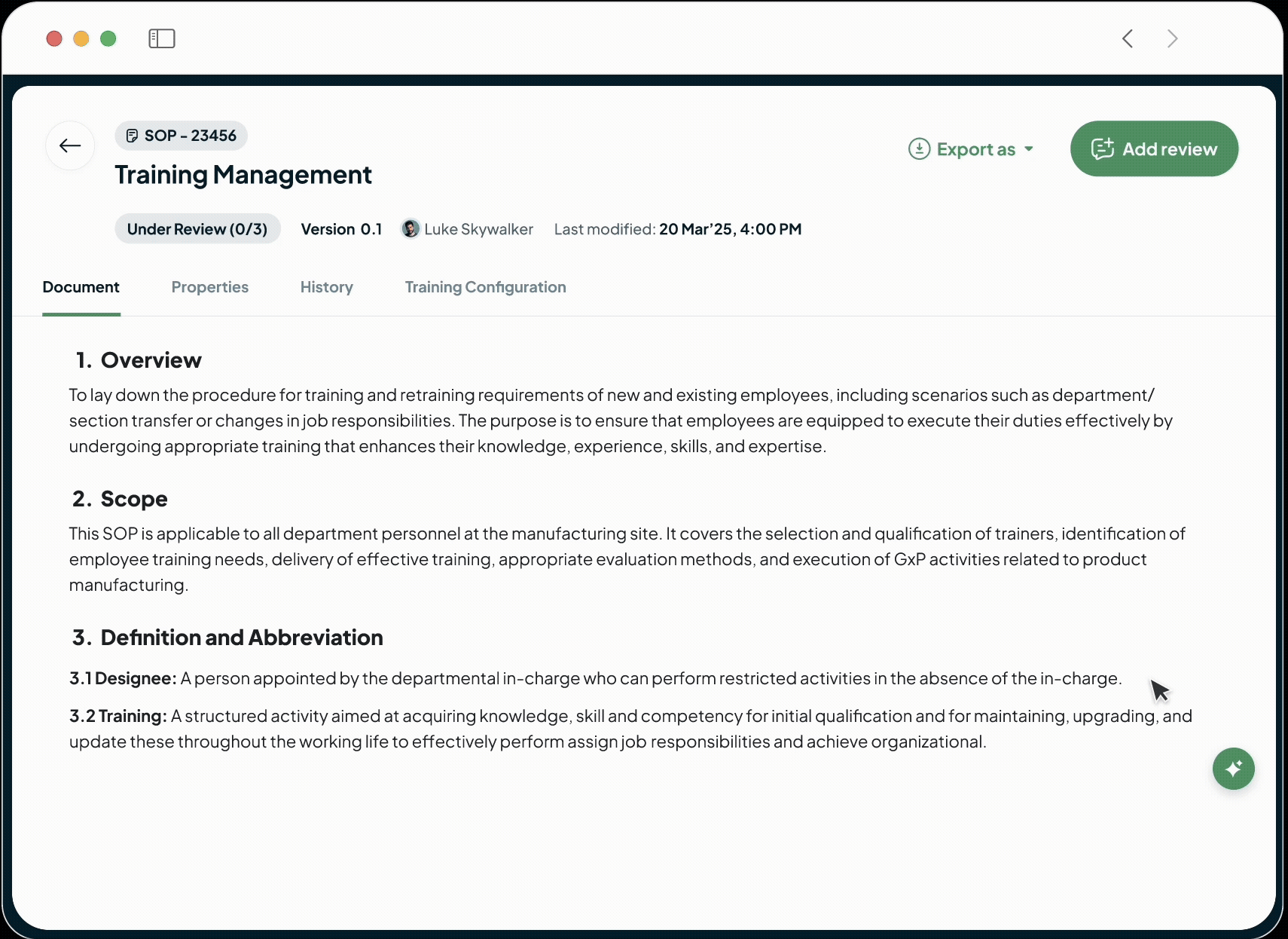
Our built-in AI-powered document editor generates clear, compliant, and consistent GxP documents from simple prompts.
Turns weeks of drafting into hours.
Ensures quality and regulatory alignment from the very first word.
Easy Collaboration
Collaborate, Review and Approve in the Same Platform
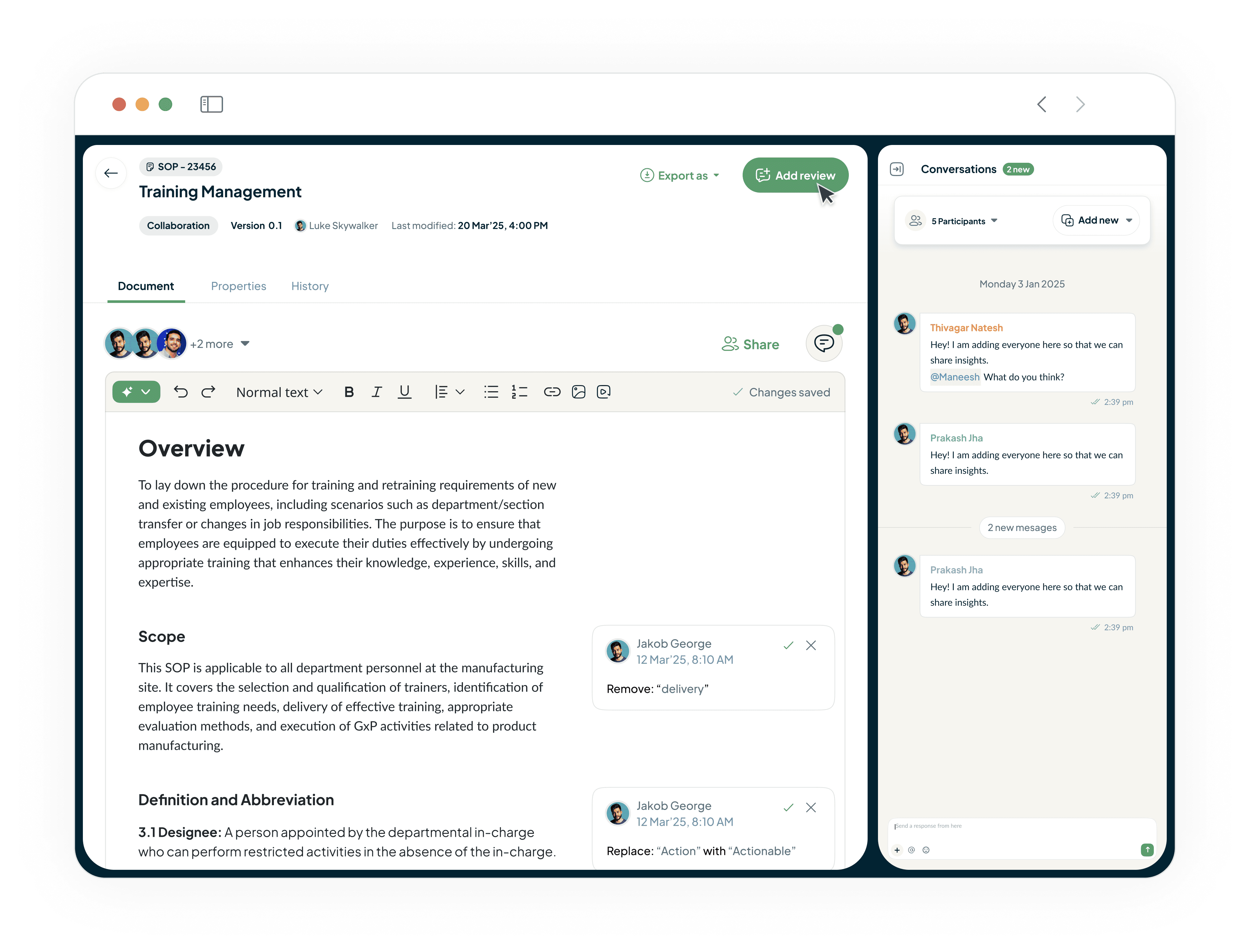
Streamline Your Entire Document Lifecycle
Our DMS platform brings your entire team together in one place.
Allows teams to comment, review, and approve documents.
Features validated e-signatures.
Cuts review cycles in half.
Intelligent Assessments
Create Assessments for Document Training Using AI
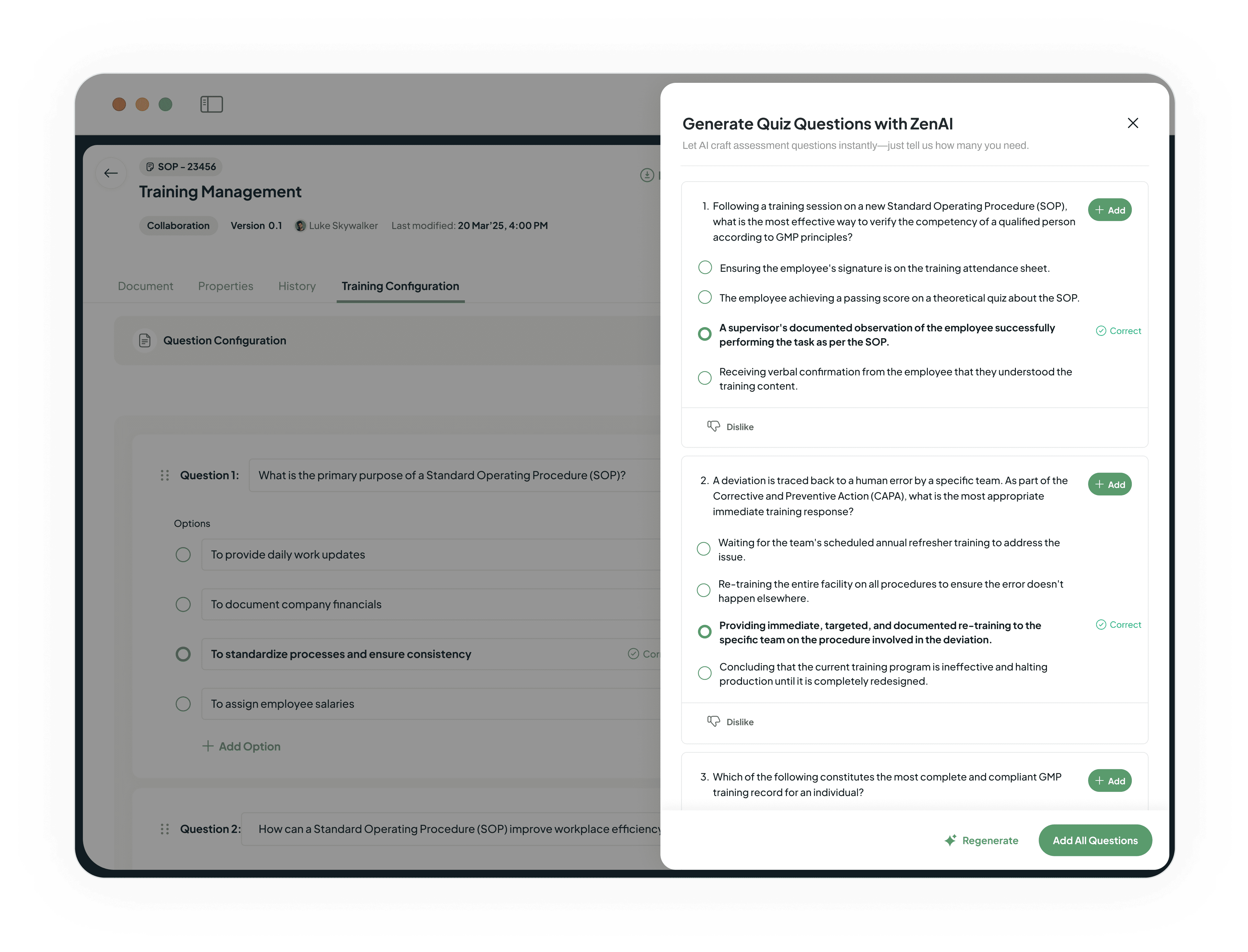
Guarantee procedural understanding
Our AI automatically generates relevant assessment questions.
Allows you to ensure and document true understanding.
Creates a formidable, audit-proof training record.
Controlled Issuance
Issue Controlled Copies and Ensure GDP and Data Integrity
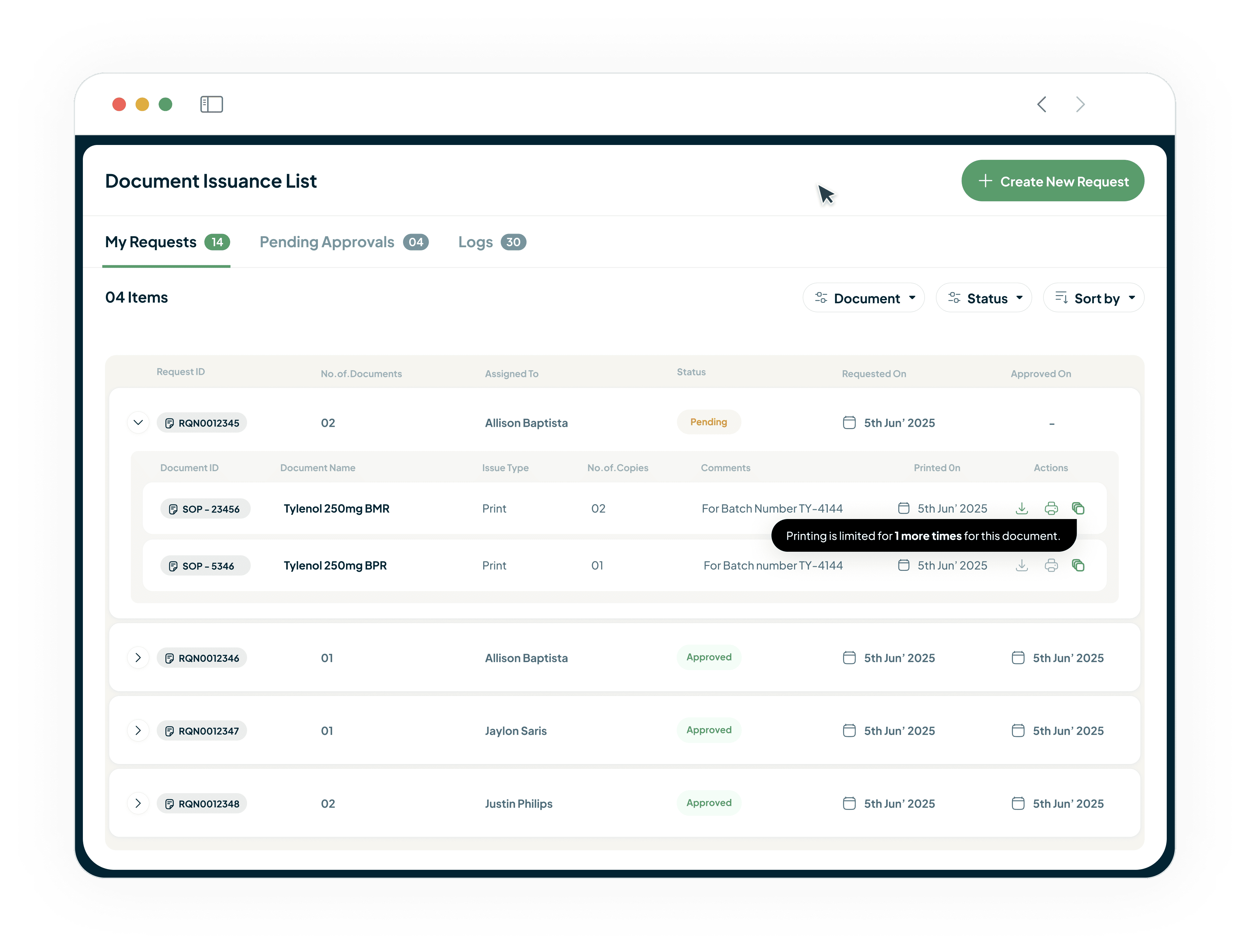
Maintain Control and Prevent Use of Obsolete Documents
Our system allows you to issue secure, watermarked, and tracked copies.
Copies can be recalled when obsolete.
Ensures Good Documentation Practices (GDP).
Safeguards both data integrity and operational quality.







