Unify ERP and GMP operations into
one AI Platform
Replace spreadsheets, paper batch records, and disconnected systems with one intelligent platform built for FDA 21 CFR Part 111 compliance. Zenopsys unifies material management, production, quality, and training—giving you real-time control over your entire operation with the efficiency of AI automation.
One AI platform for global compliance
Testimonials
What Industry Leaders are Saying
Unify ERP, quality and compliance into one AI platform
Purpose built for Life sciences
Why teams switch from spreadsheets and legacy software to Zenopsys
Legacy / Paper
Outdated methods
- Doesn't scale
- High cost for customisations
- Zero visibility
- Lack of Integrations
- High cost of operations
- lengthy manual processes
- AI cannot be implemented
Enterprise Software
Overcomplicated
- Heavy customisation required
- Long Implementation timeline
- Siloed Systems
- Expensive Integrations
- High cost per Seat
- Rigid processes
- Data isn't AI-ready
Zenopsys
Optimized solution
Ready-to-use, user-friendly modules
Go live in 15 days
Unified Platform
In built Integrations
Flat pricing
Flexible processes with AI automation
AI native platform
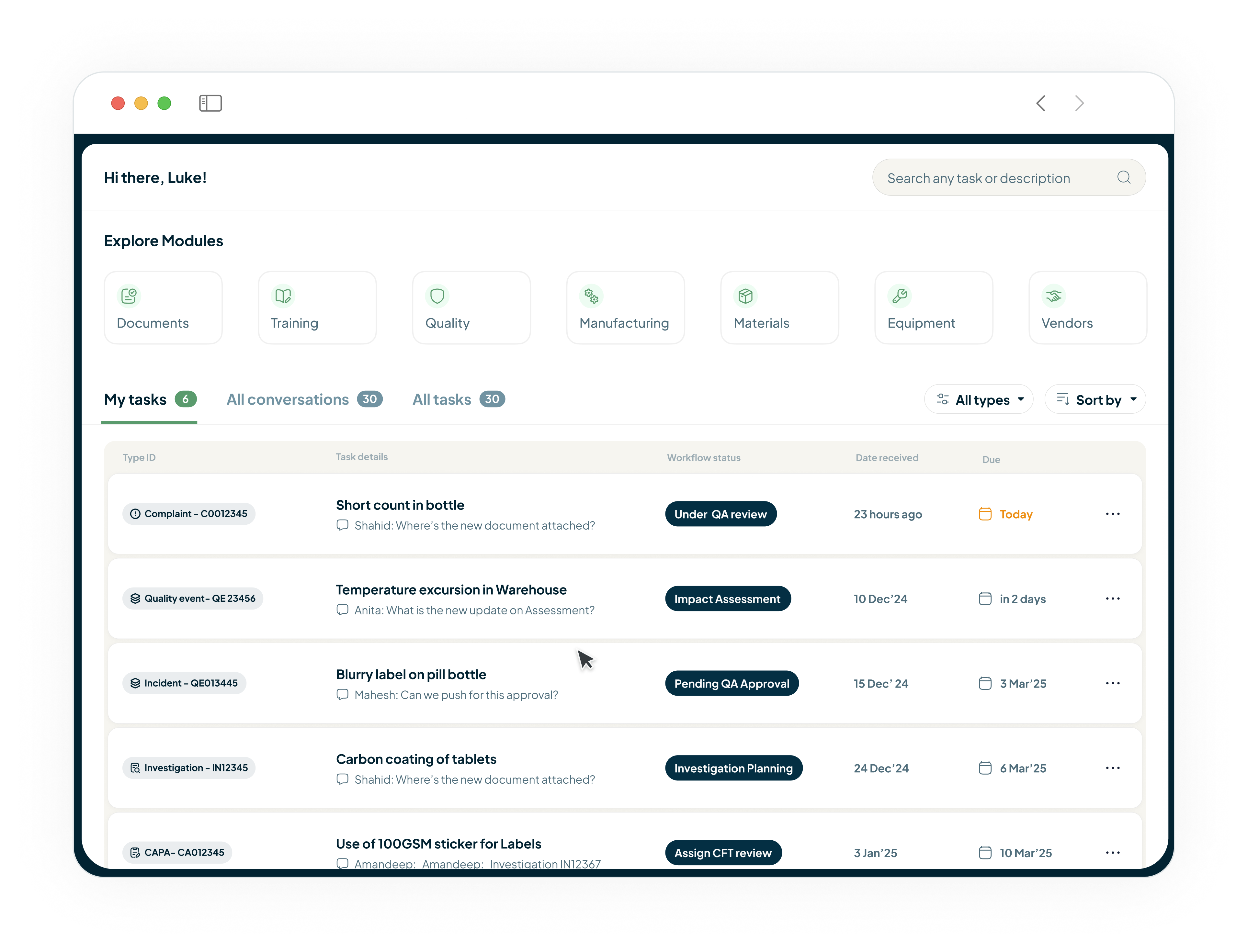
Achieve Total Compliance with FDA 21 CFR Part 111
Our platform is built with FDA 21 CFR Part 111 and BRC Global requirements at its core. From 21 CFR Part 11 compliant e-signatures and audit trails on every document and quality event to GxP-compliant training records, be audit ready with confidence, knowing your records are secure, traceable, and instantly accessible.
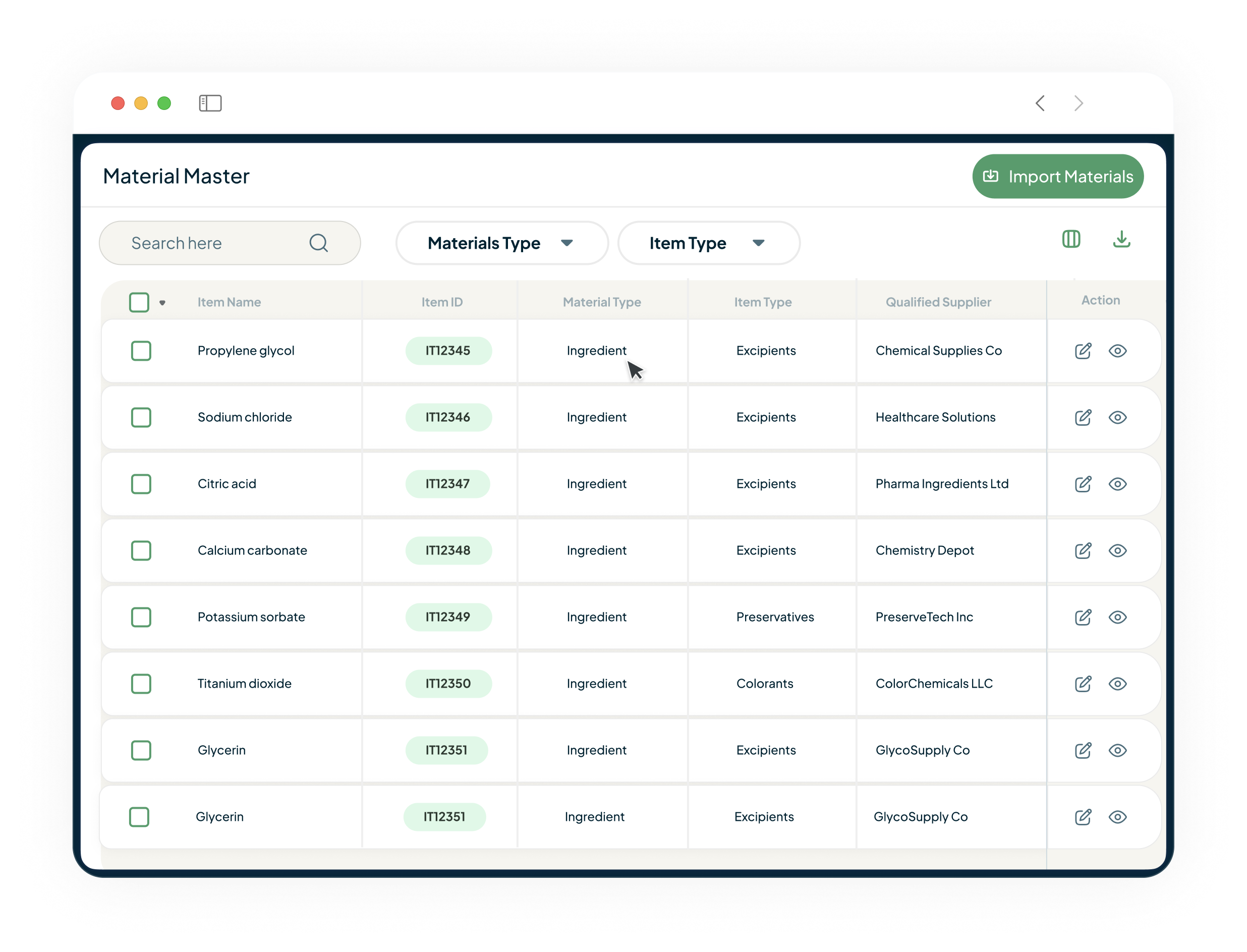
Gain End-to-End Control of Your Supply Chain
Connect your suppliers, inventory, and production floor. Our platform unifies Vendor Management, Material Management, and Production Planning to give you total control of final product.
- Vendors: Qualify, audit, and manage your critical raw ingredient suppliers to ensure compliance and quality before materials arrive.
- Inventory: Get real-time visibility into your warehouse, manage material specifications, and track inventory from inwarding to wip.
- Planning: Use Material Requirement Planning (MRP) and batch scheduling to ensure you have the right ingredients and resources ready to meet demand
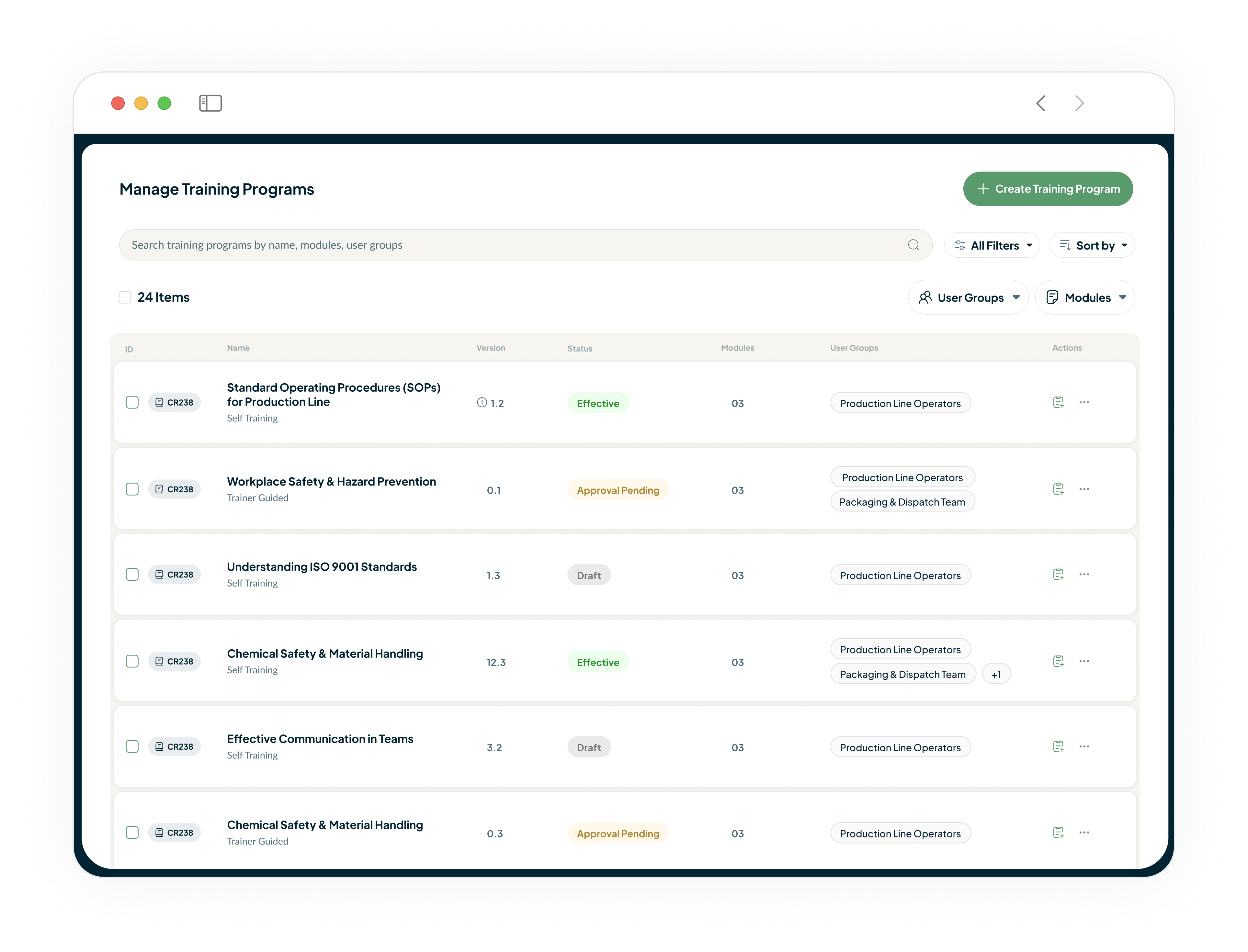
Training Records for GMP Compliance
Our Learning Management System automatically assigns training when documents change, tracks completion with time-stamped records, and provides instant audit reports showing your entire team's training history.
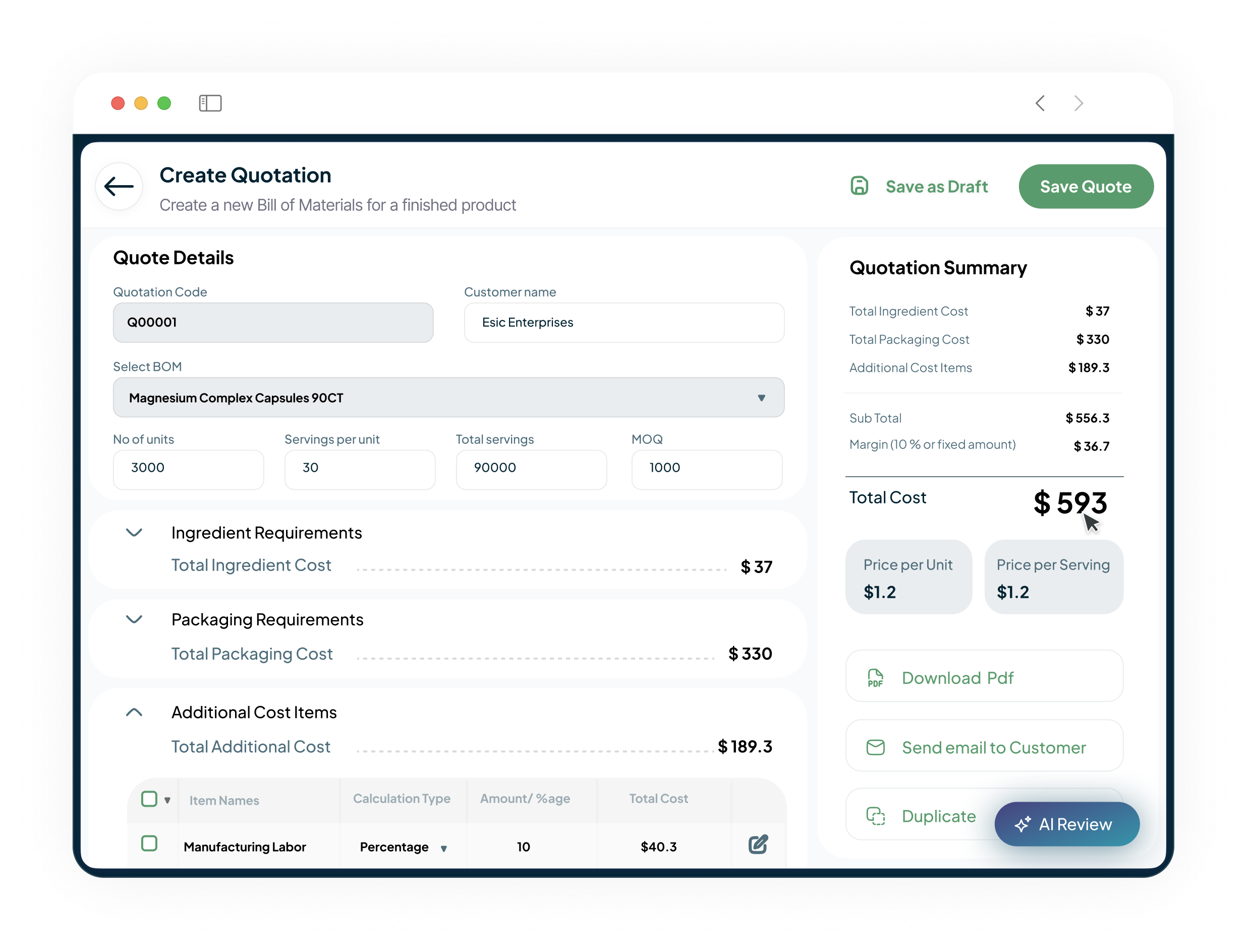
Rapid Product Costing & Quote Generation
Our Quotation Management module calculates costs instantly using current ingredient prices, labour, and overhead. Generate professional quotes in minutes, not days, and convert accepted quotes to production orders automatically, accelerating the path to revenue.
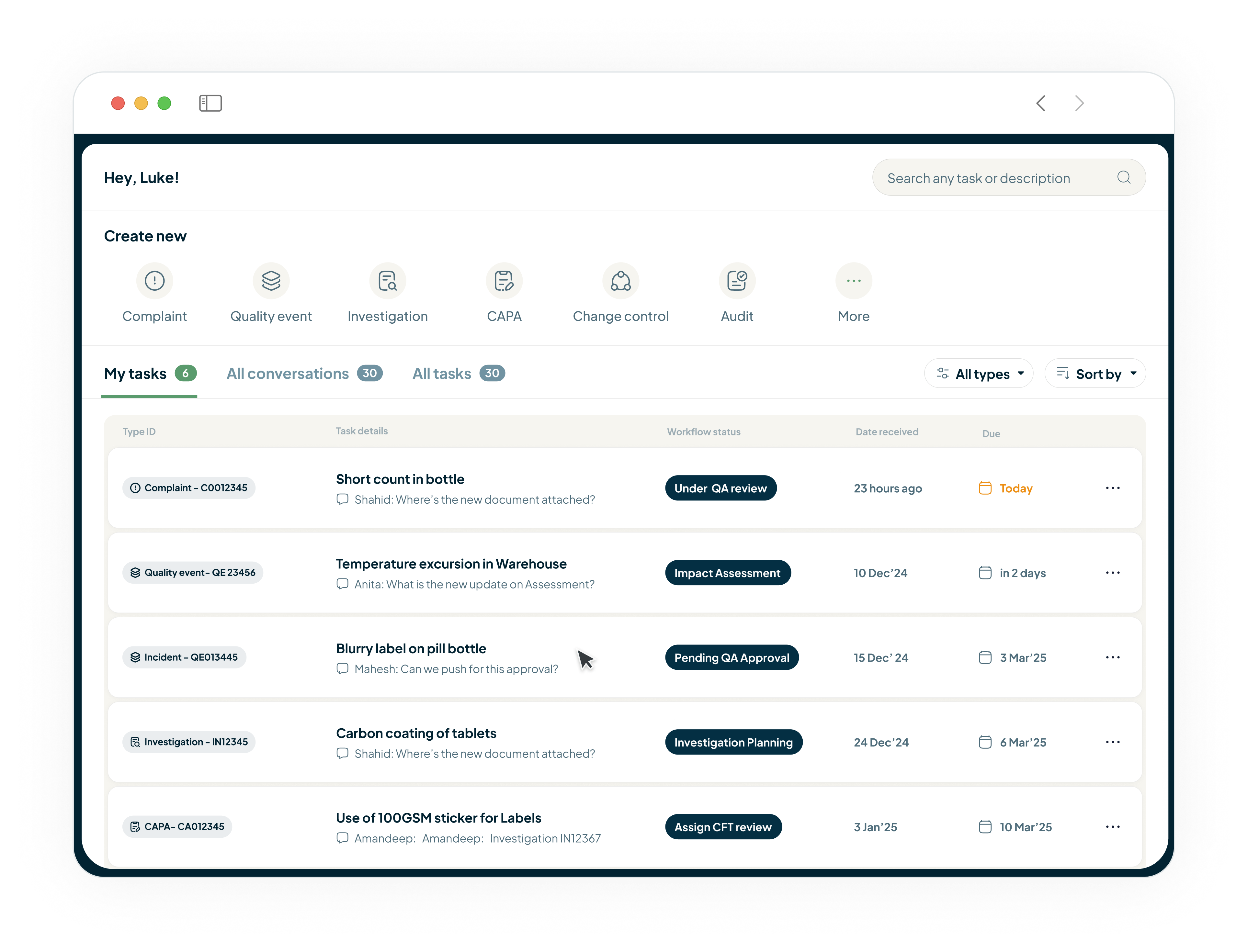
Complaint Investigation & CAPA Closure
Our QMS provides structured workflows that guide your team through root cause analysis, automatically link complaints to specific production batches, and track CAPAs through closure.
