Unify ERP and GMP operations into
one AI Platform
Zenopsys unifies your formulas, quality systems, and production in one AI-native platform—accelerating batch release by 80%, eliminating manual errors, and giving you audit-ready traceability in seconds.
What Industry Leaders are Saying
Unify ERP, quality and compliance into one AI platform
Why teams switch from spreadsheets and legacy software to Zenopsys
Legacy / Paper
Outdated methods
- Doesn't scale
- High cost for customisations
- Zero visibility
- Lack of Integrations
- High cost of operations
- lengthy manual processes
- AI cannot be implemented
Enterprise Software
Overcomplicated
- Heavy customisation required
- Long Implementation timeline
- Siloed Systems
- Expensive Integrations
- High cost per Seat
- Rigid processes
- Data isn't AI-ready
Zenopsys
Optimized solution
Ready-to-use, user-friendly modules
Go live in 15 days
Unified Platform
In built Integrations
Flat pricing
Flexible processes with AI automation
AI native platform
MoCRA Compliance Requirements & Solutions
Manually tracking and submitting serious adverse event (SAE) reports leads to delays, incomplete filings, and costly penalties.
Digitally capture, track, and submit SAE reports with proactive alerts for MoCRA 15-day deadlines never miss a compliance window.
Maintaining years of product and safety data across paper and spreadsheets risks audit failure and enforcement actions.
Zenopsys archives safety and product records electronically for 6+ years, all easily retrievable for regulators.
Linking adverse events to batches is fragmented, making root cause analysis slow and recall response slow.
Instantly link events, batches, and ingredients supporting rapid root cause, audit defense, and recall readiness.
GMP Principles Requirements & Solutions
SOPs, change controls, and batch records are scattered, causing loss of control and increased deviation risk.
Centralized, version-controlled SOPs and batch records ensure GDP enforcement, change tracking, and batch integrity.
Paper-based or manual training has gaps, risking unqualified personnel in production and failed audits.
Automated, role-based team training with e-signature tracking and audit-ready records, validated to 21 CFR Part 11.
Manual documentation delays CAPA and deviation closure, driving repeat issues and higher scrutiny.
Automated digital CAPA workflows with AI-powered root cause suggestions and real-time status dashboards.
ISO 22716 Standards Requirements & Solutions
Fragmented quality systems make it hard to enforce global standards and reduce ISO 22716 audit observations.
Unify all quality management in a single system aligned to ISO 22716, with instant global traceability and record access.
Inadequate version control and documentation lead to breakdowns in Good Documentation Practice (GDP).
Every document is digitally versioned, watermarked, tamper-proof, and linked to approval and training status.
Disconnected supplier and material management increases the risk of out-of-spec ingredients and missed certifications.
Track, approve, and audit every supplier, batch, and certificate digitally, ensuring ingredient quality and compliance at every stage.
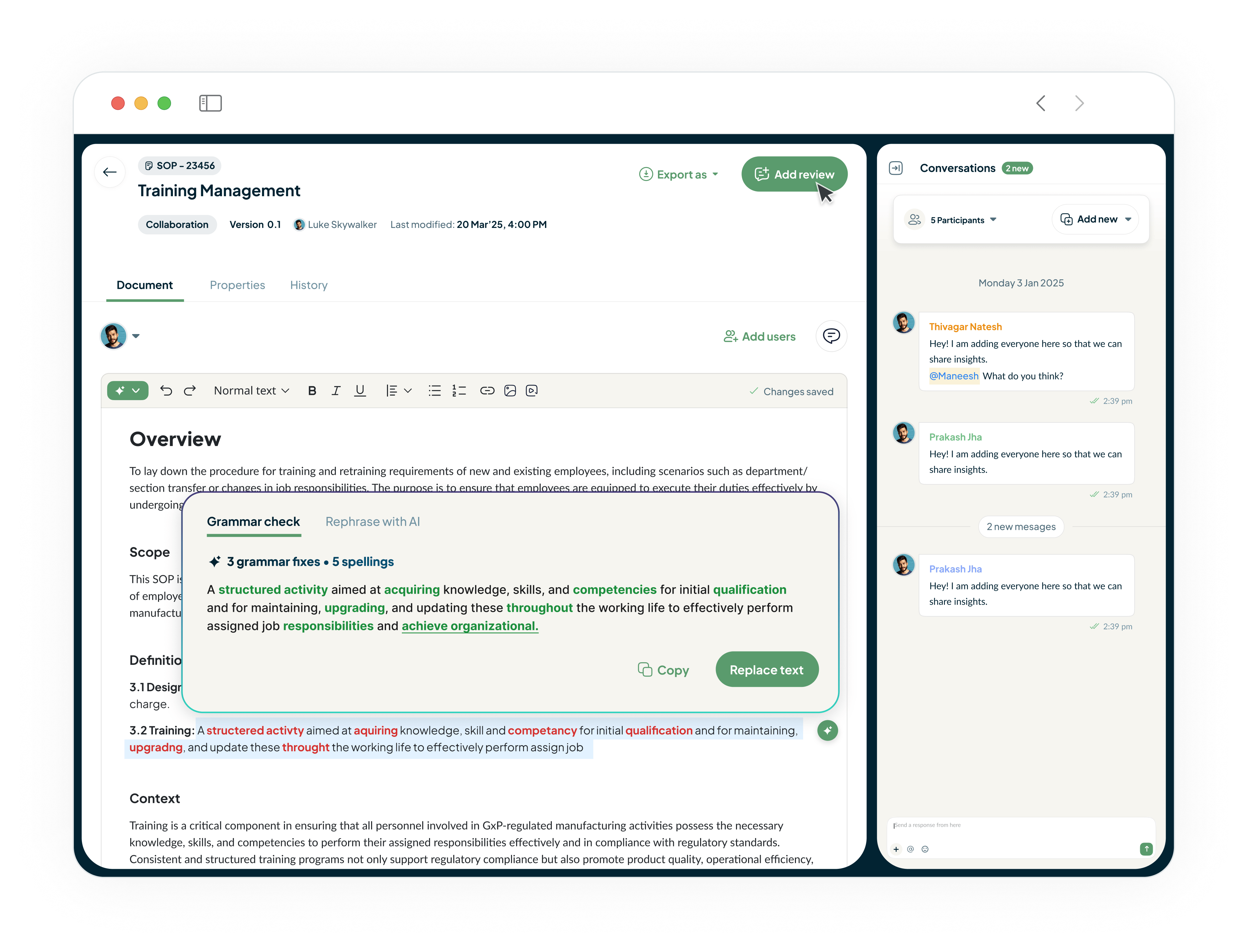
Achieve and Maintain ISO 22716 & MoCRA Compliance
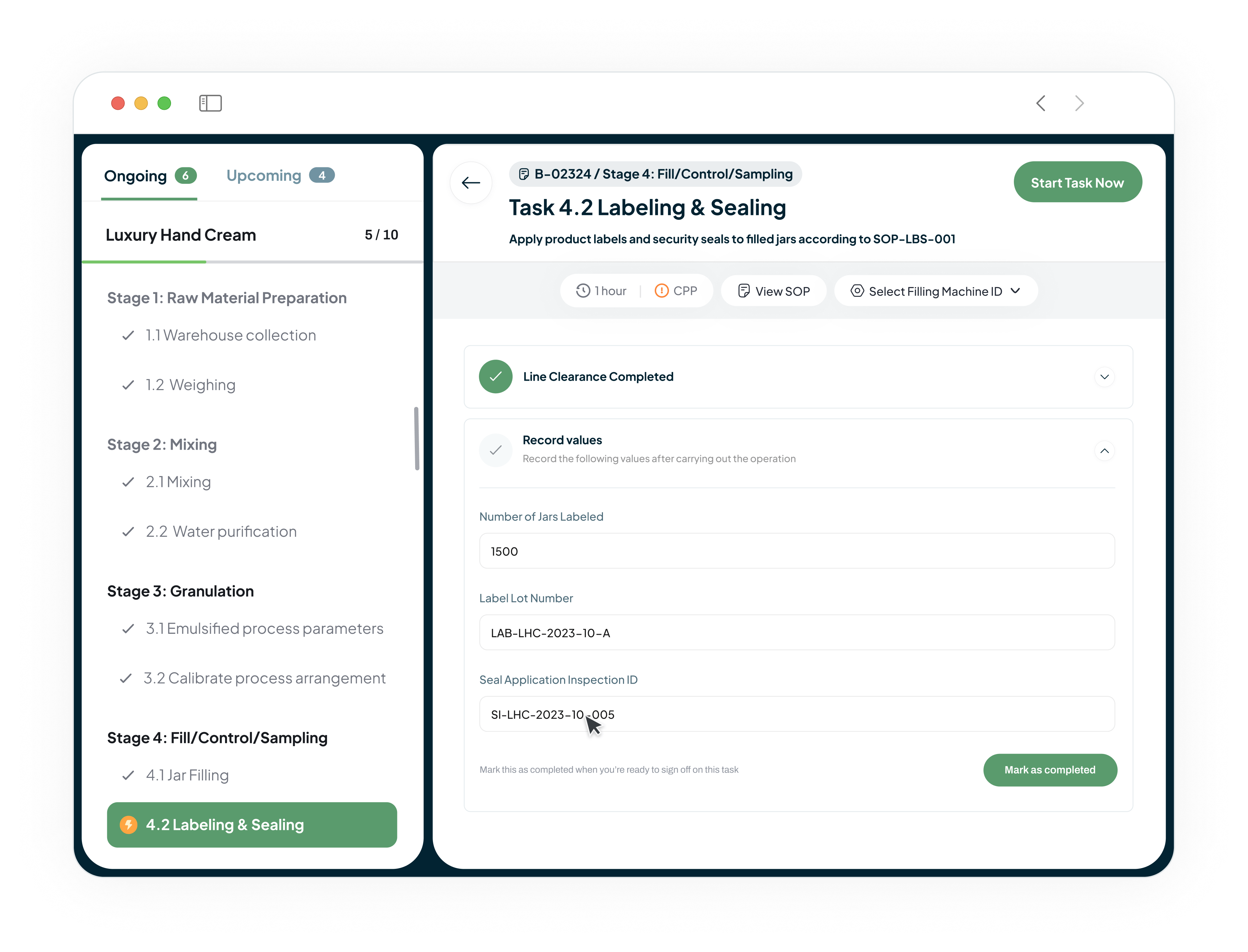
Digitize Batch Records and Accelerate Product Release
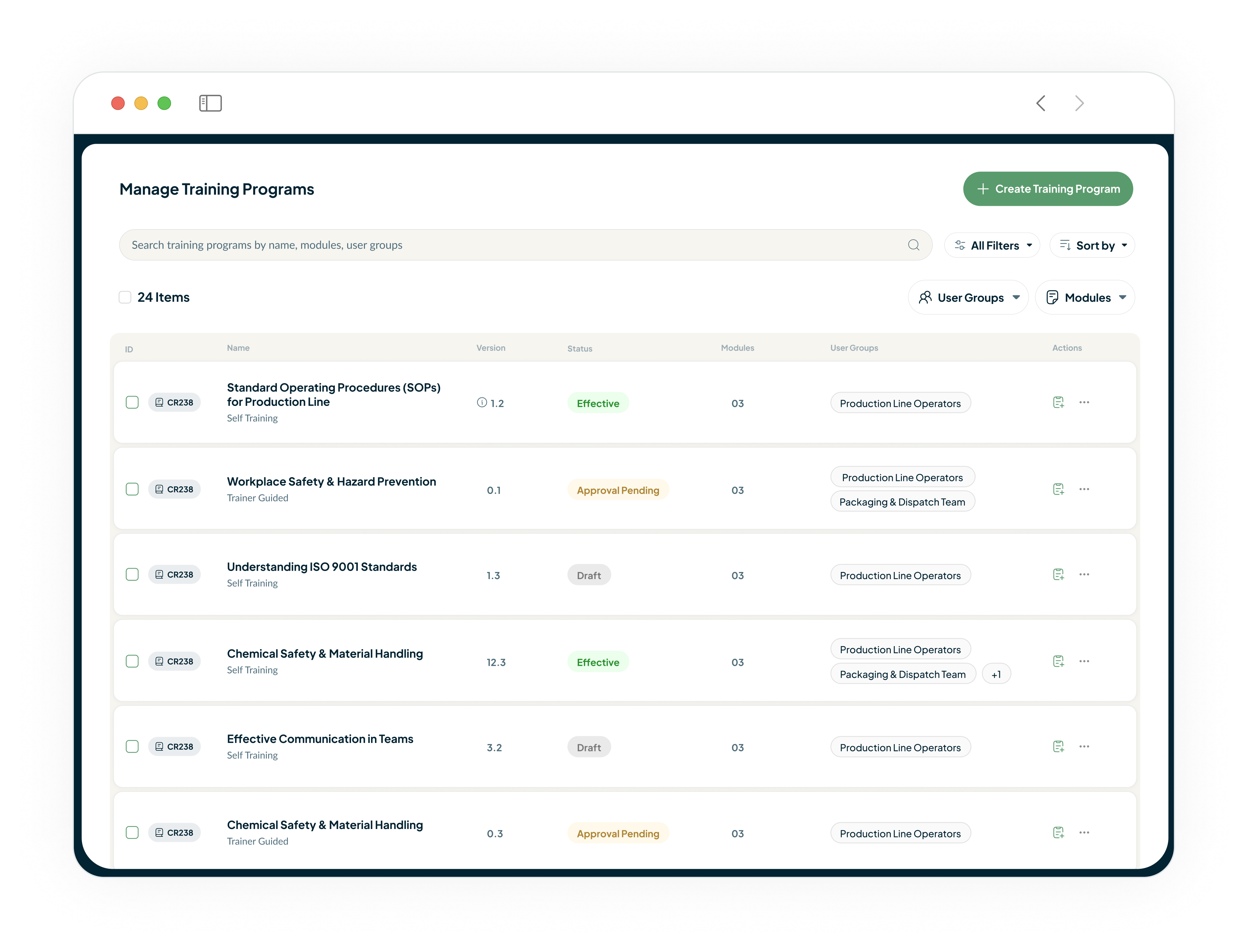
Automate Your GMP Training and Close Compliance Gaps
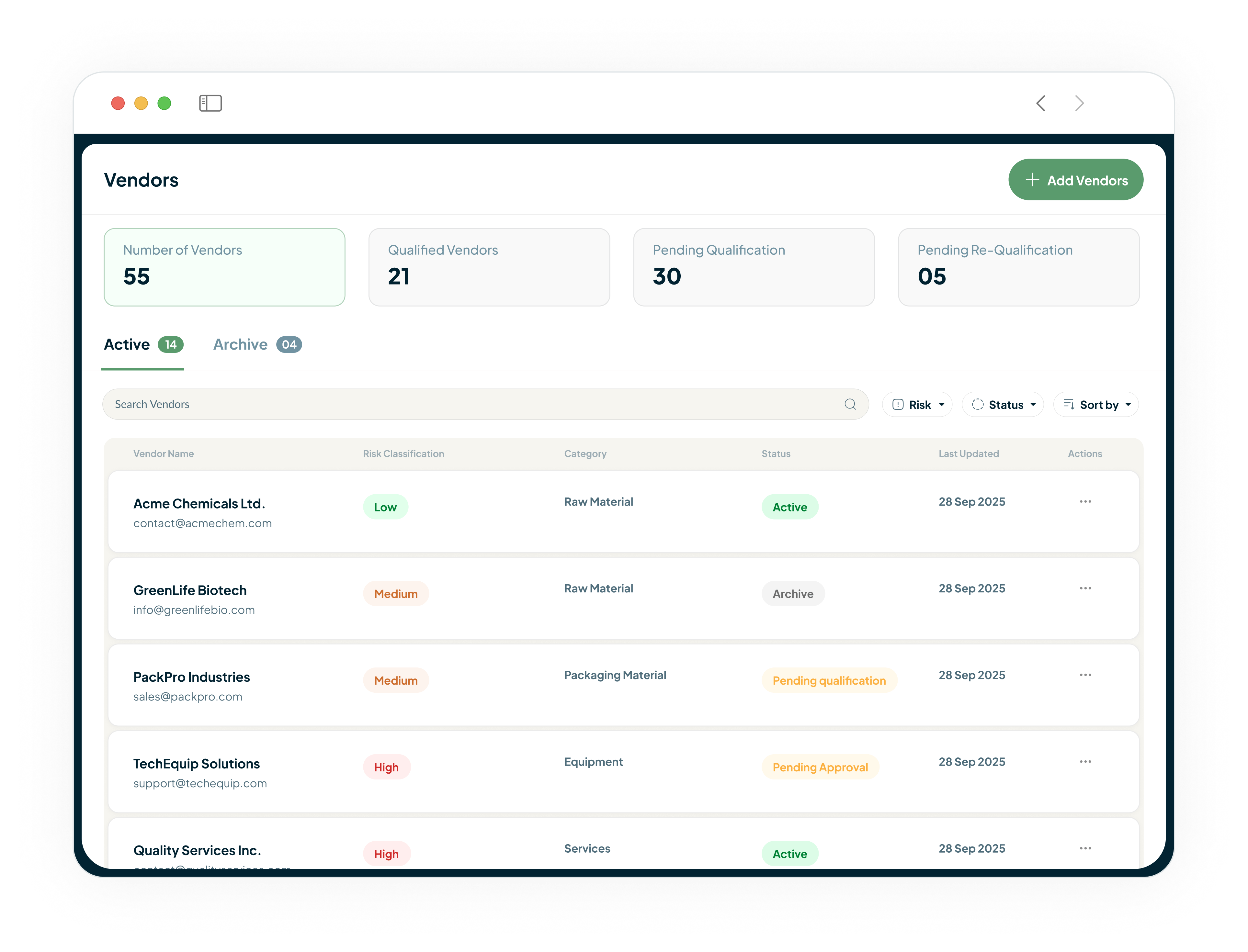
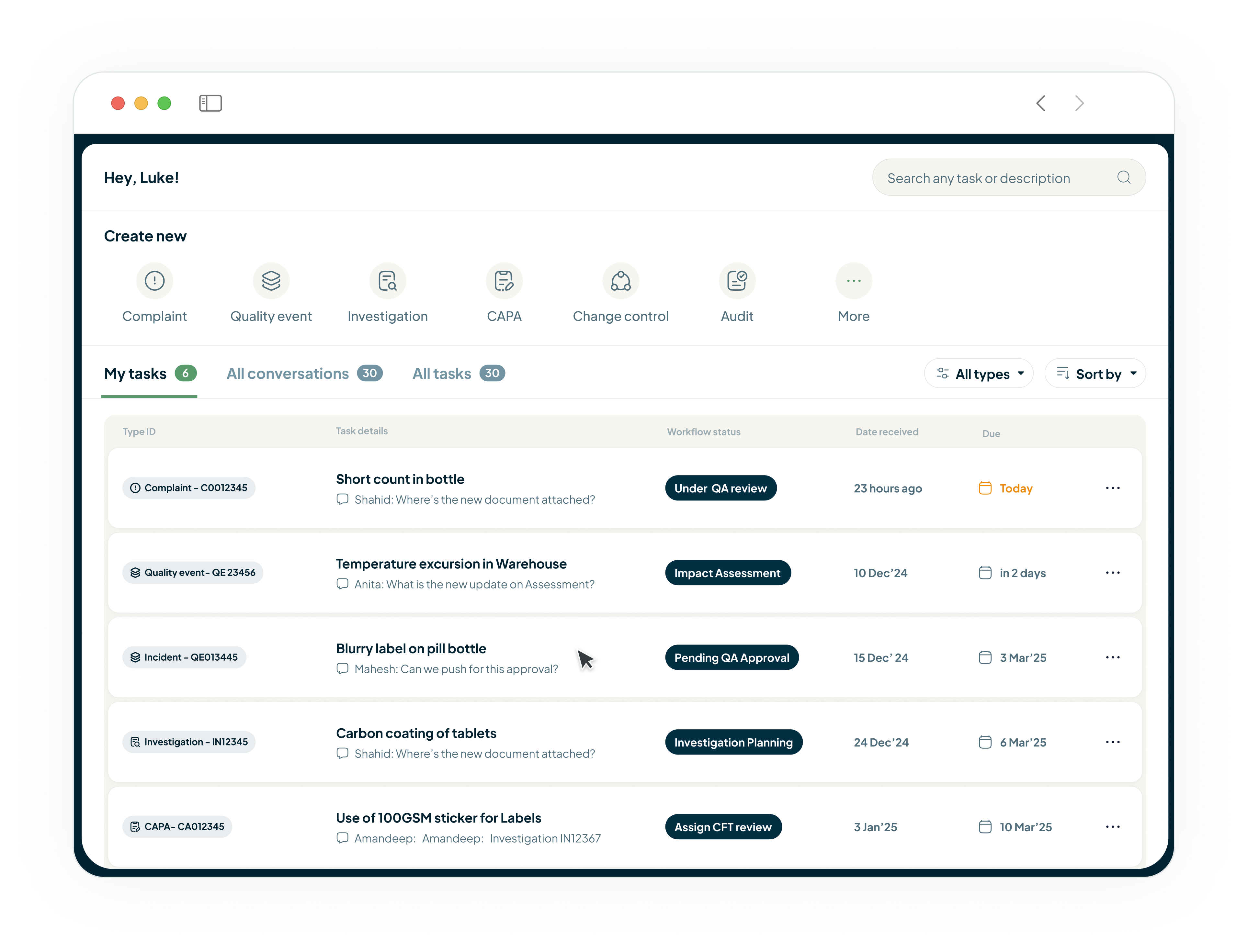
Gain End-to-End Control of Your Materials and Supply Chain