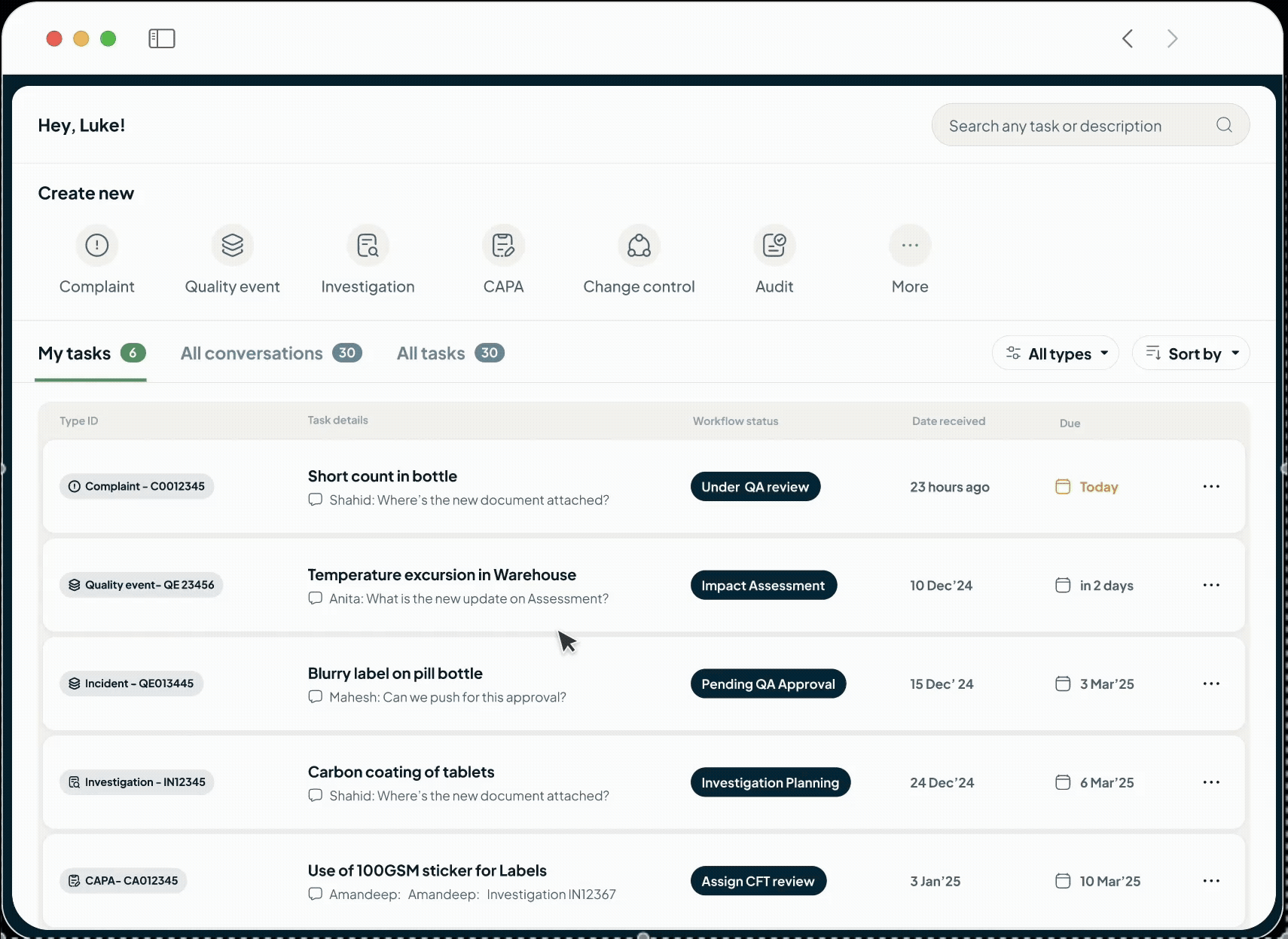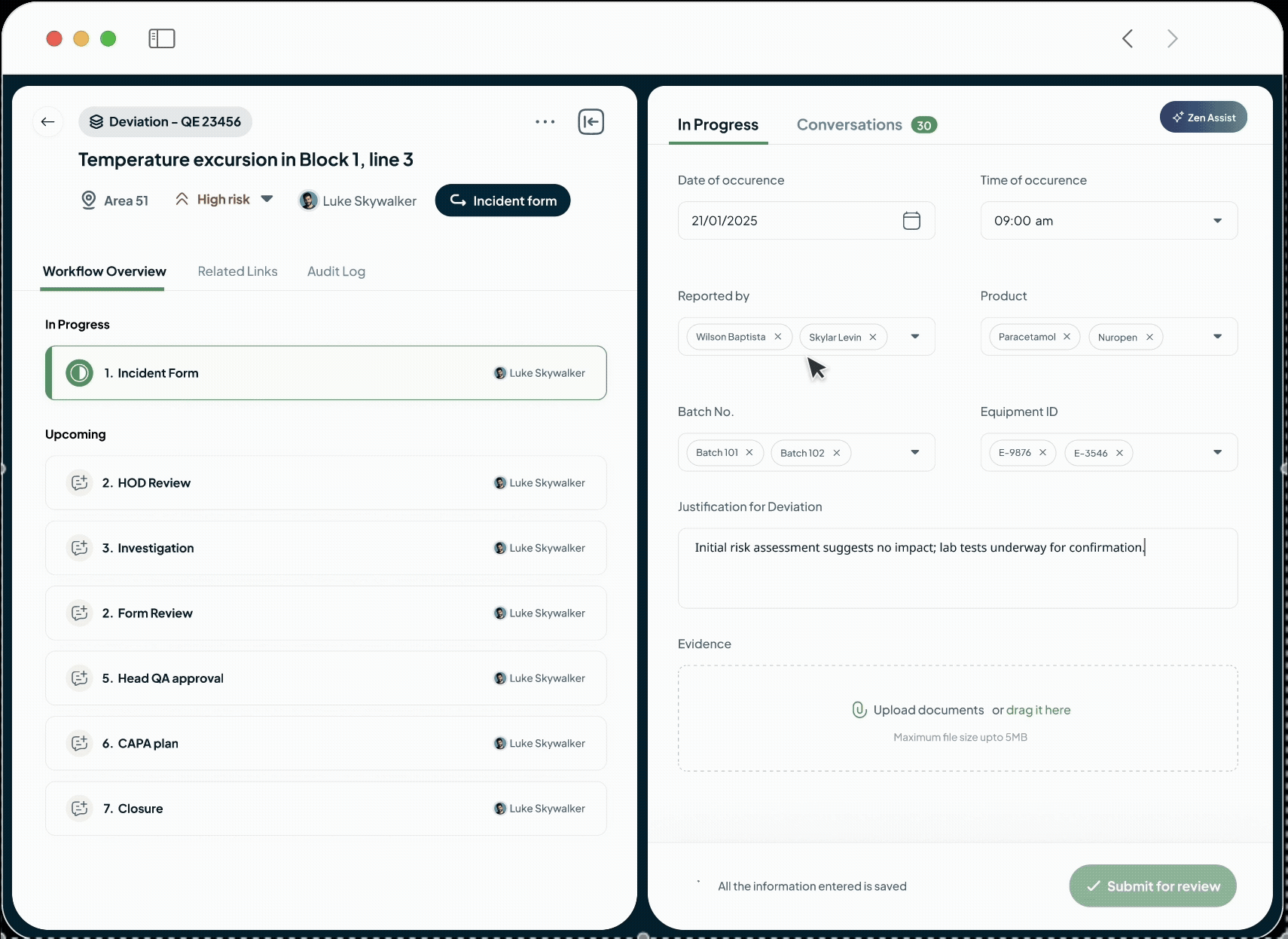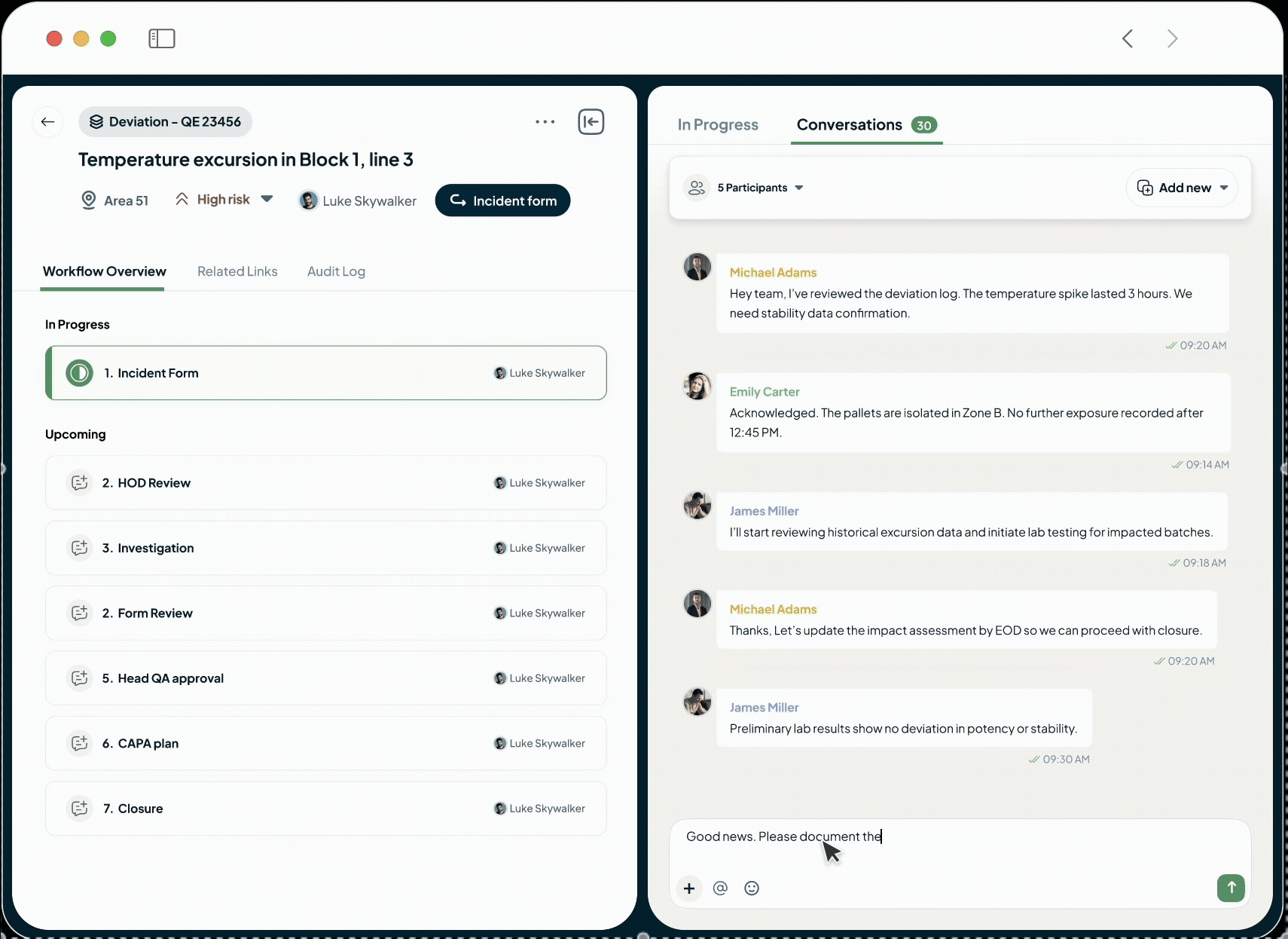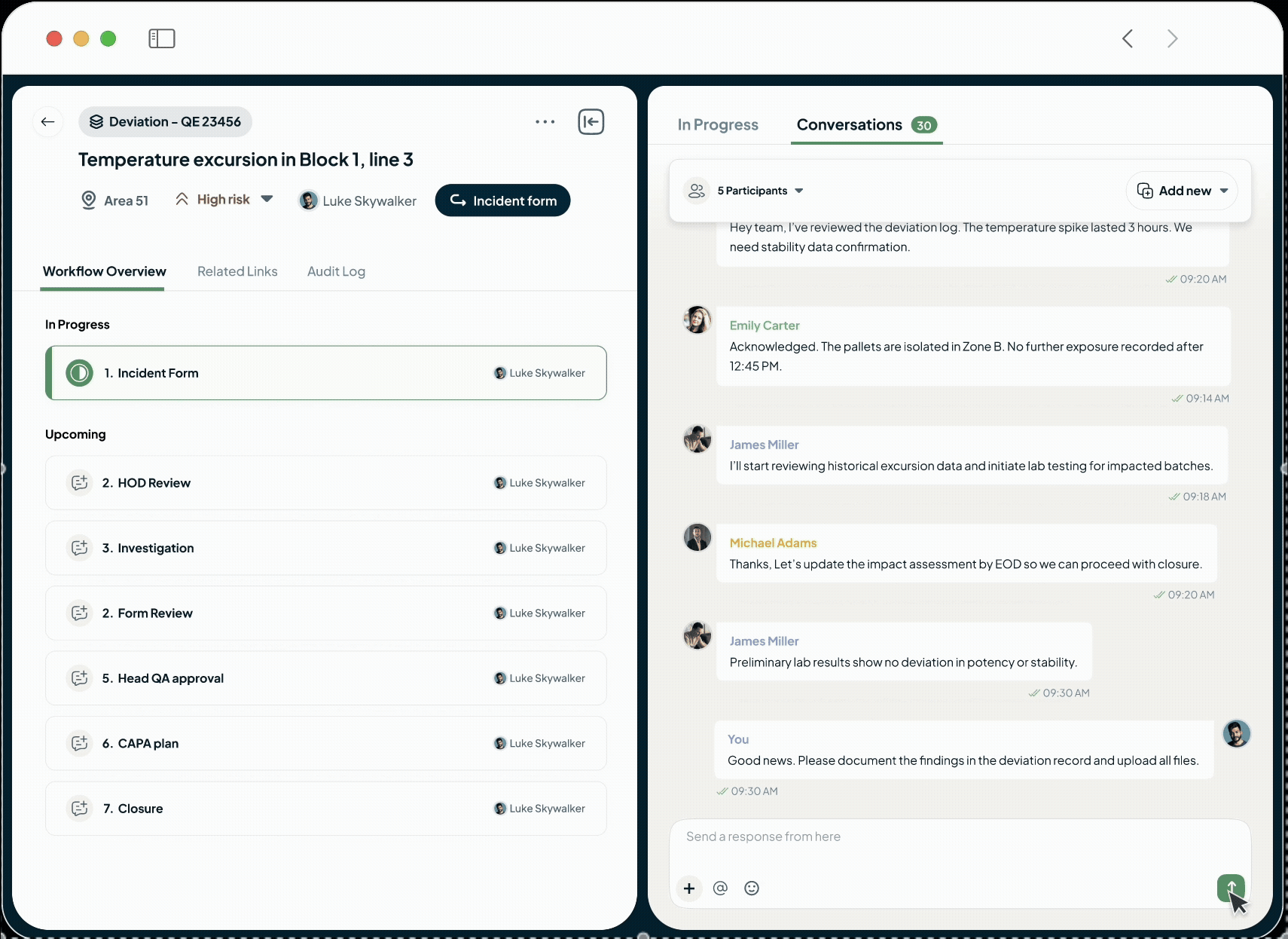
Unify GxP operations into
one AI Platform
We're an intelligent, operational backbone for the modern life sciences manufacturer, built by founders who have walked your factory floors and faced your auditors.


200+ years of industry expertise empowering us
Backed by two centuries of proven expertise, we bring unmatched knowledge and reliability to every solution.
Backed by the best in the industry

CUSTOMERS WE SERVE

One AI Powered Platform
From RnD to Commercial Operations
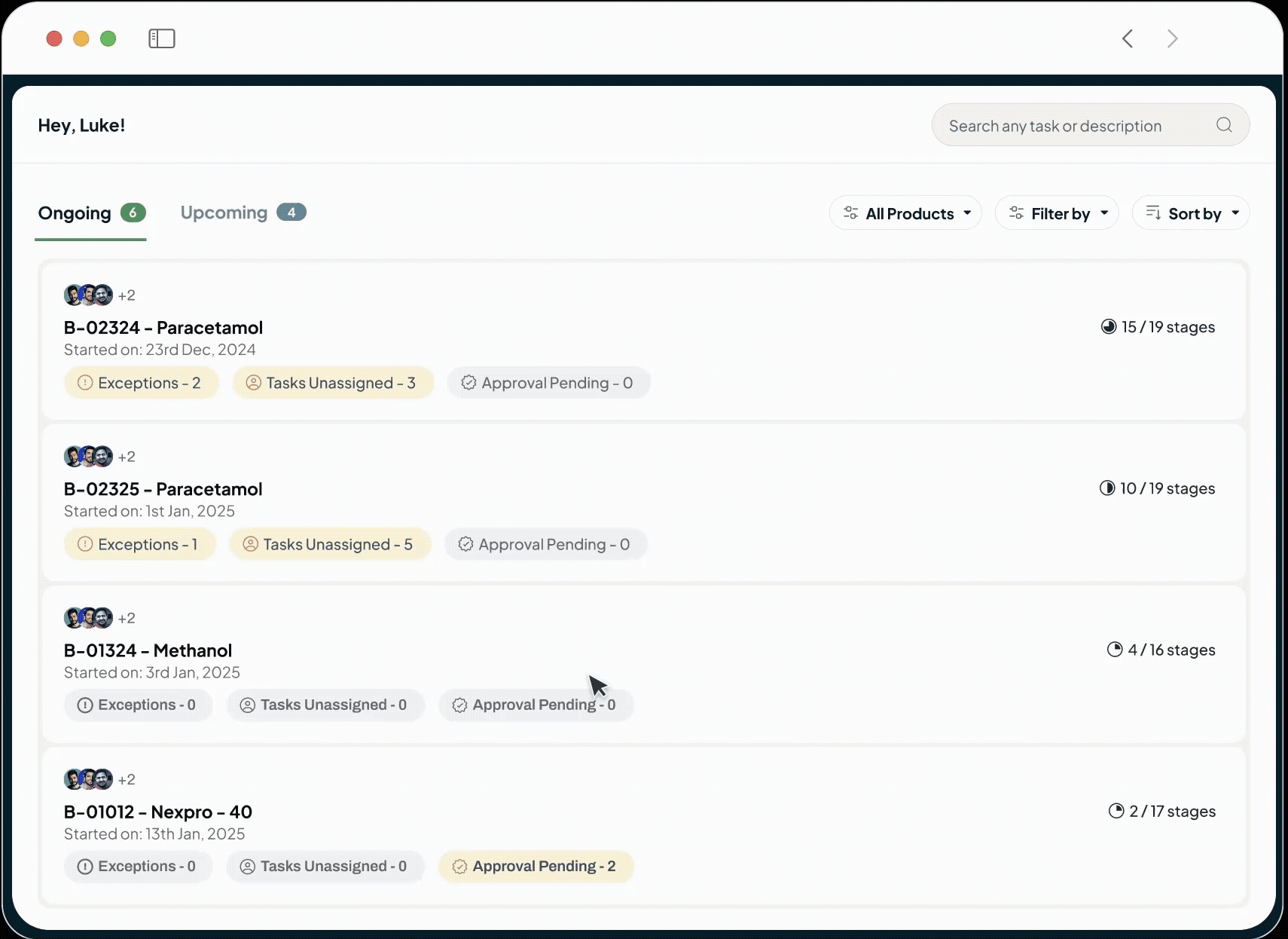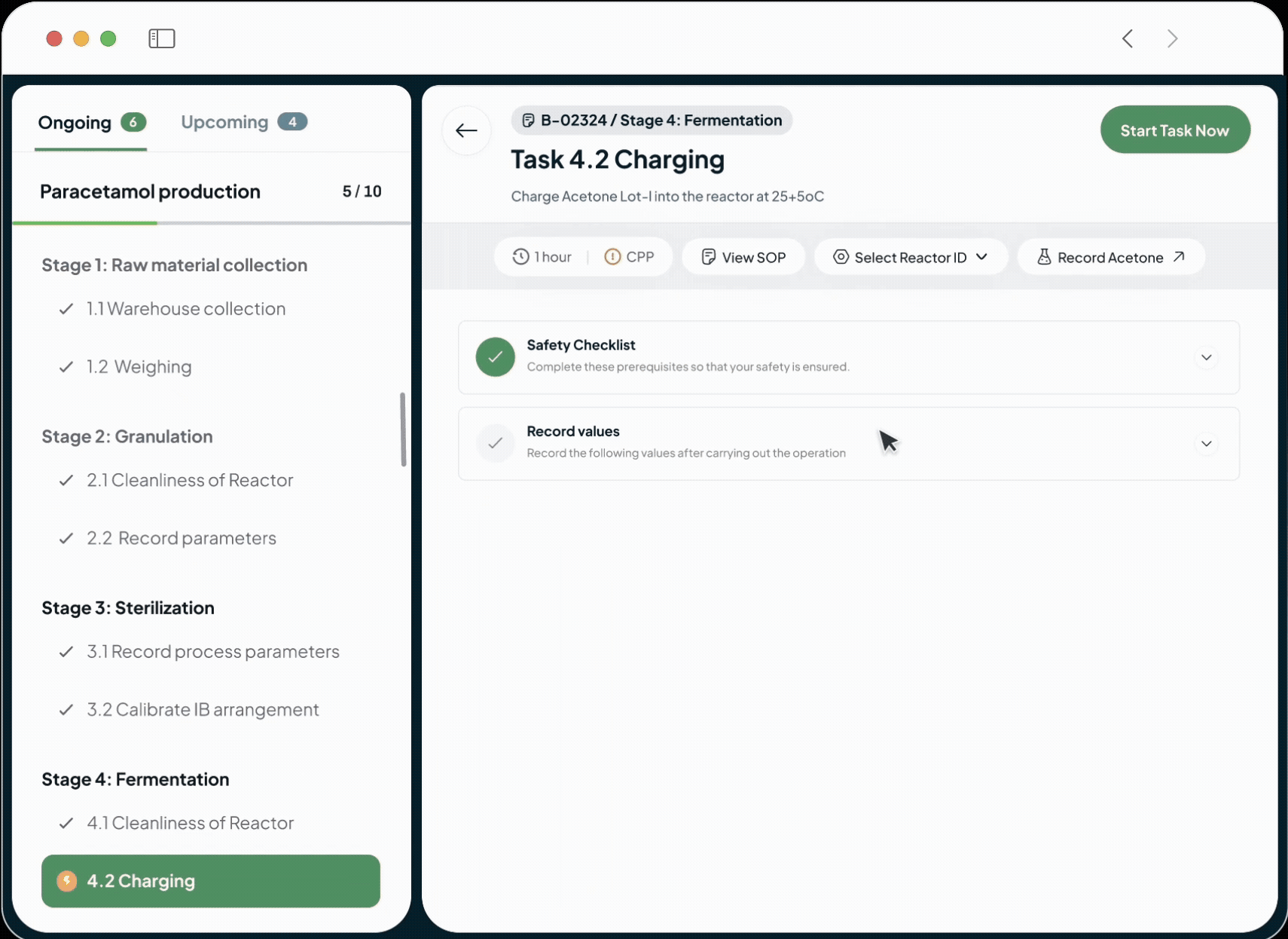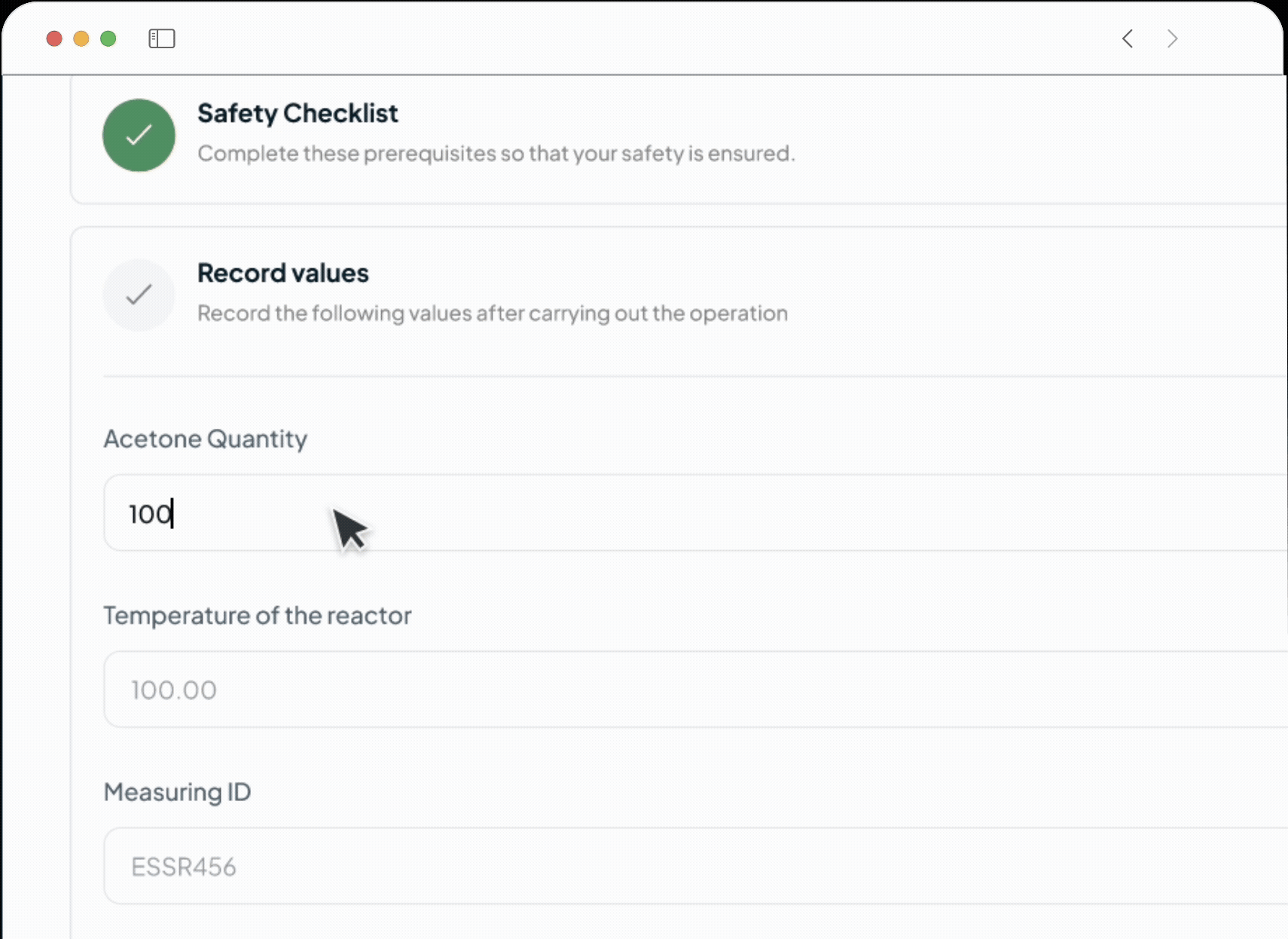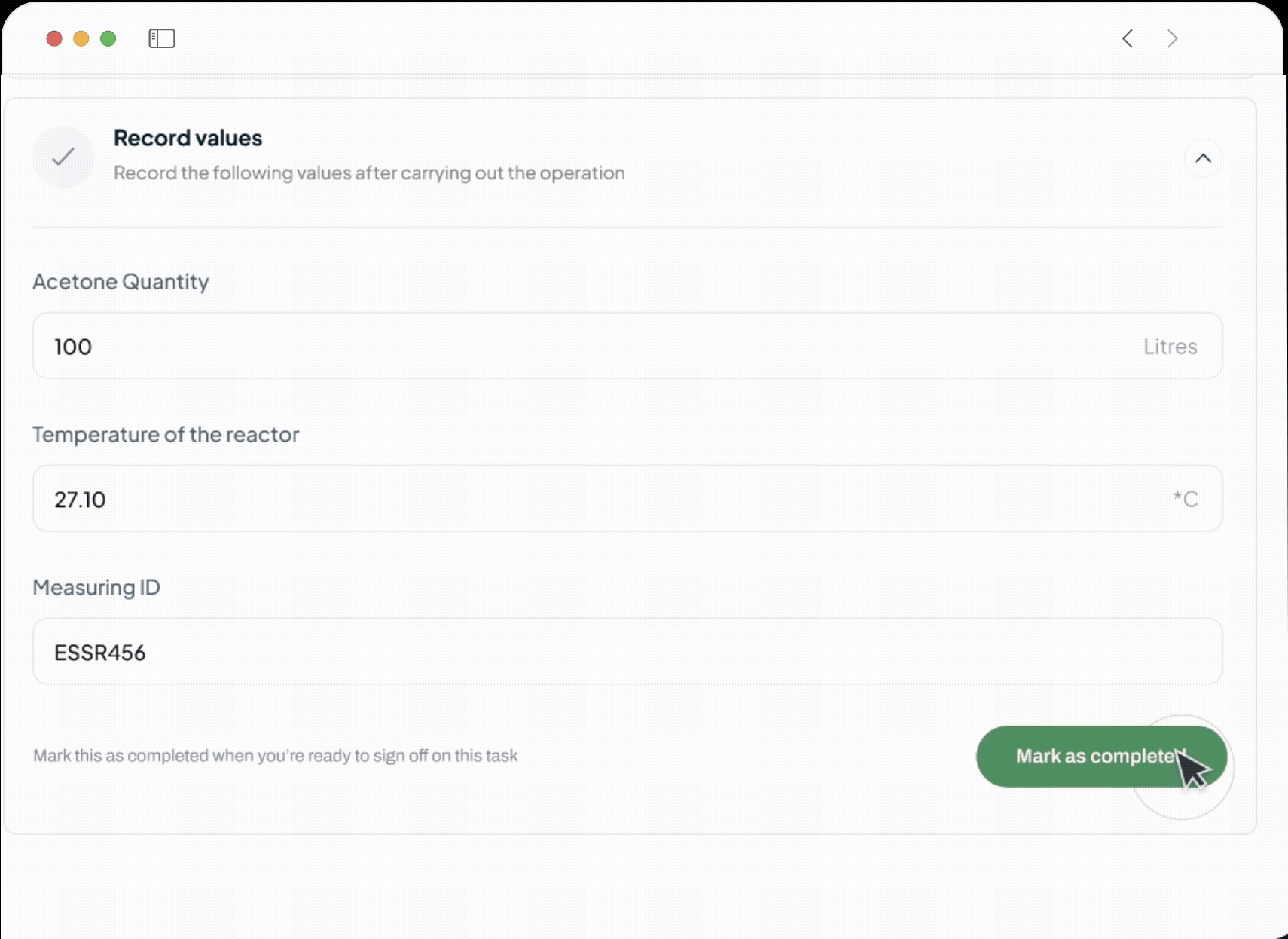
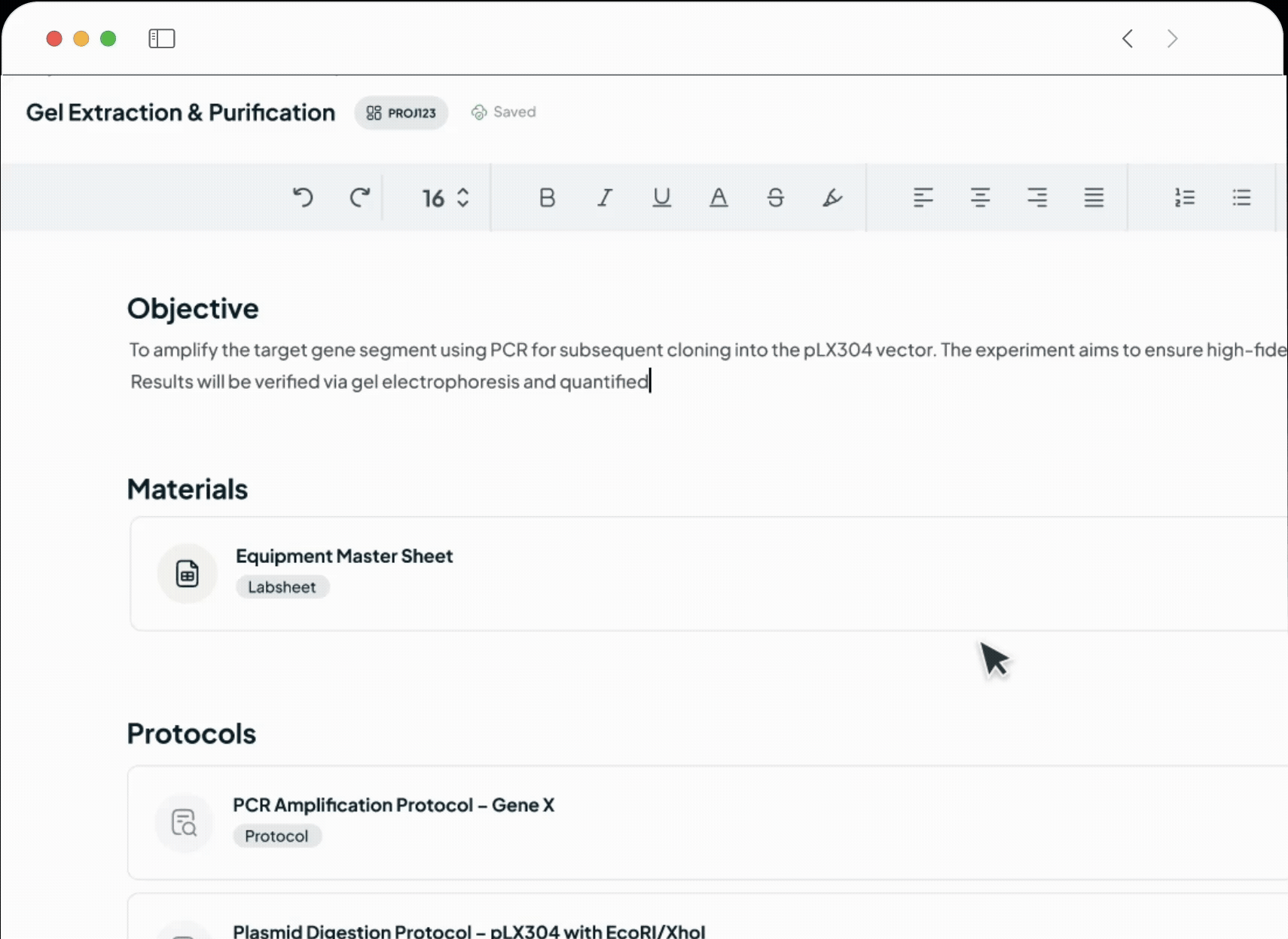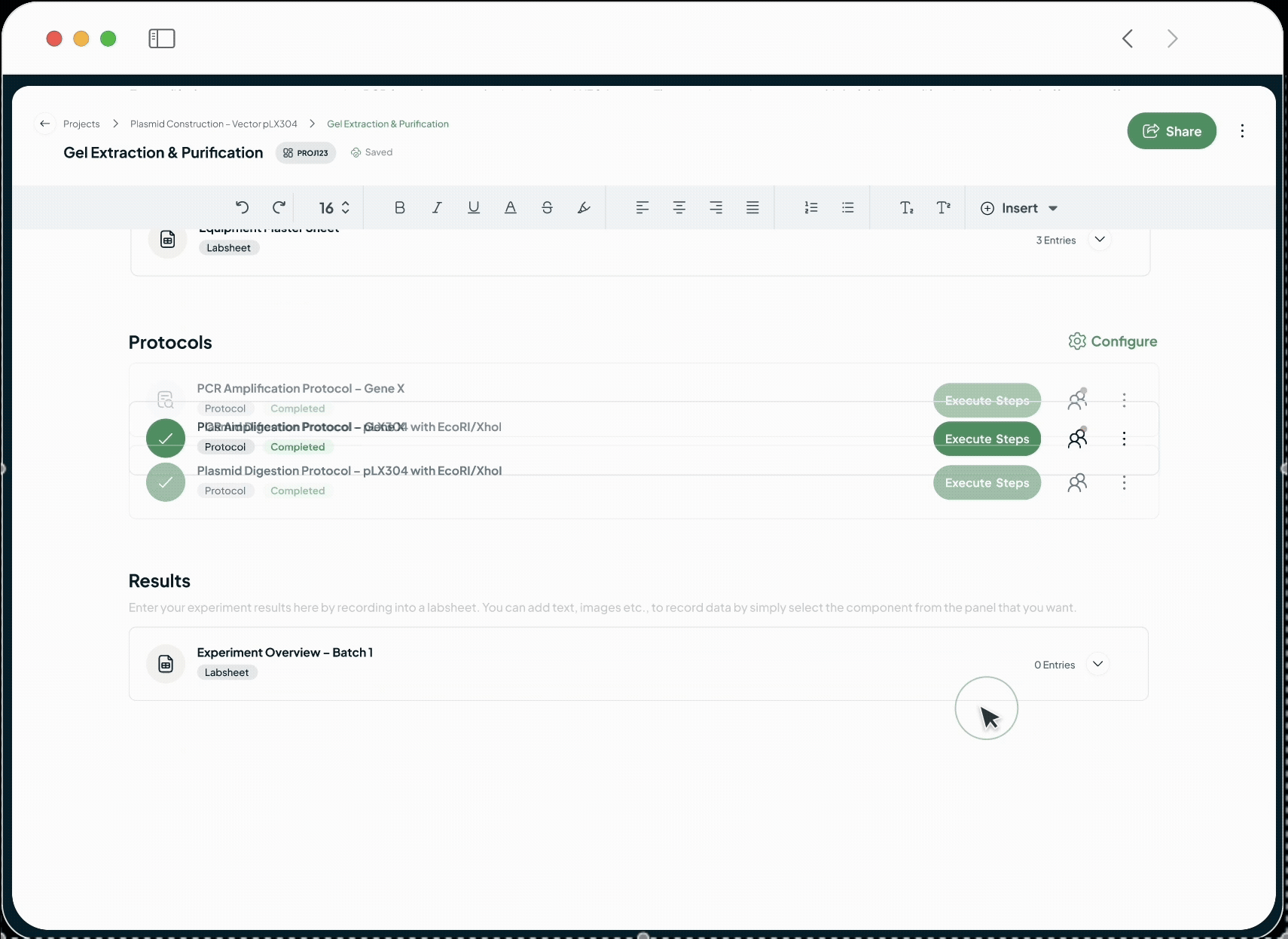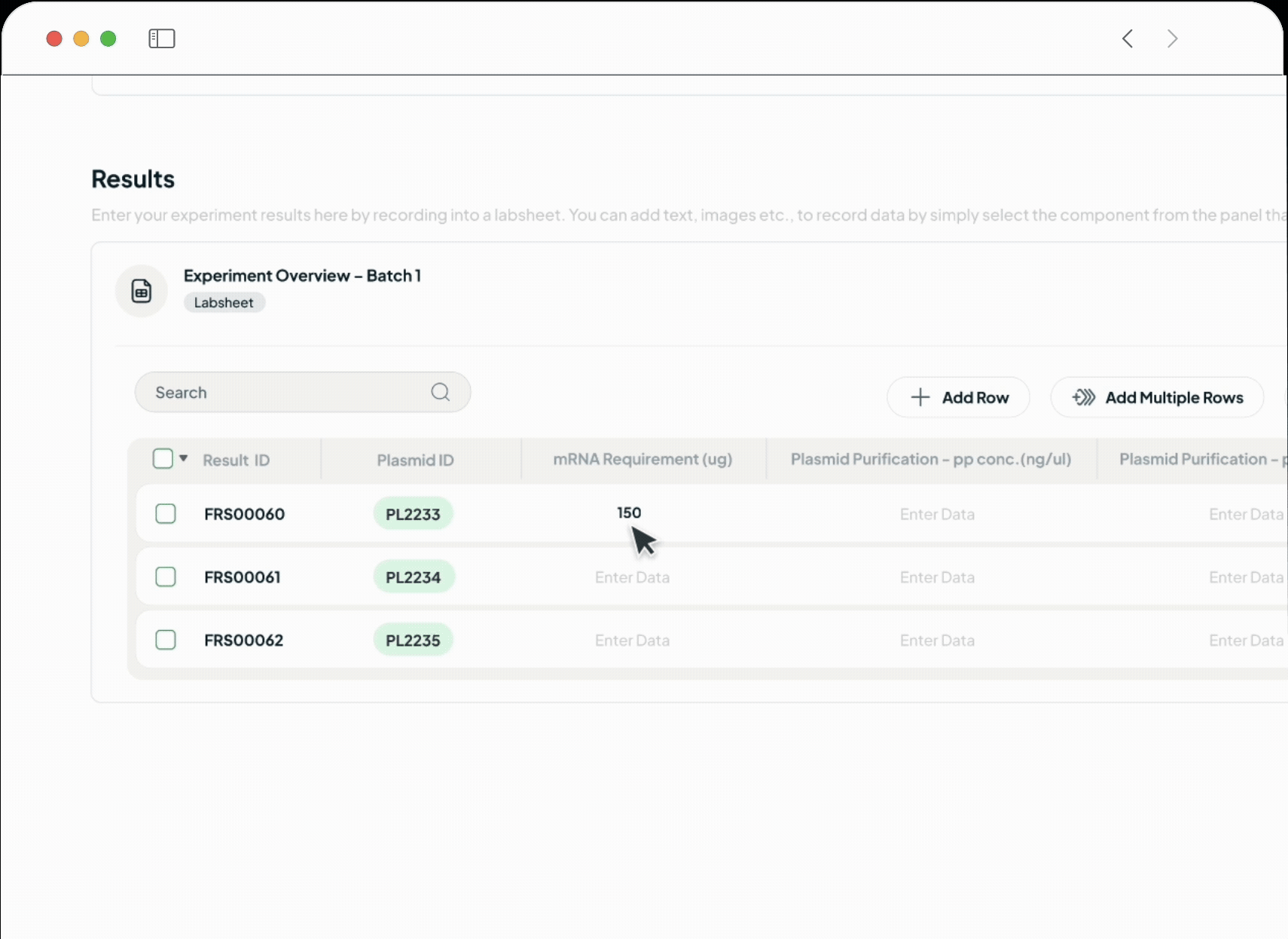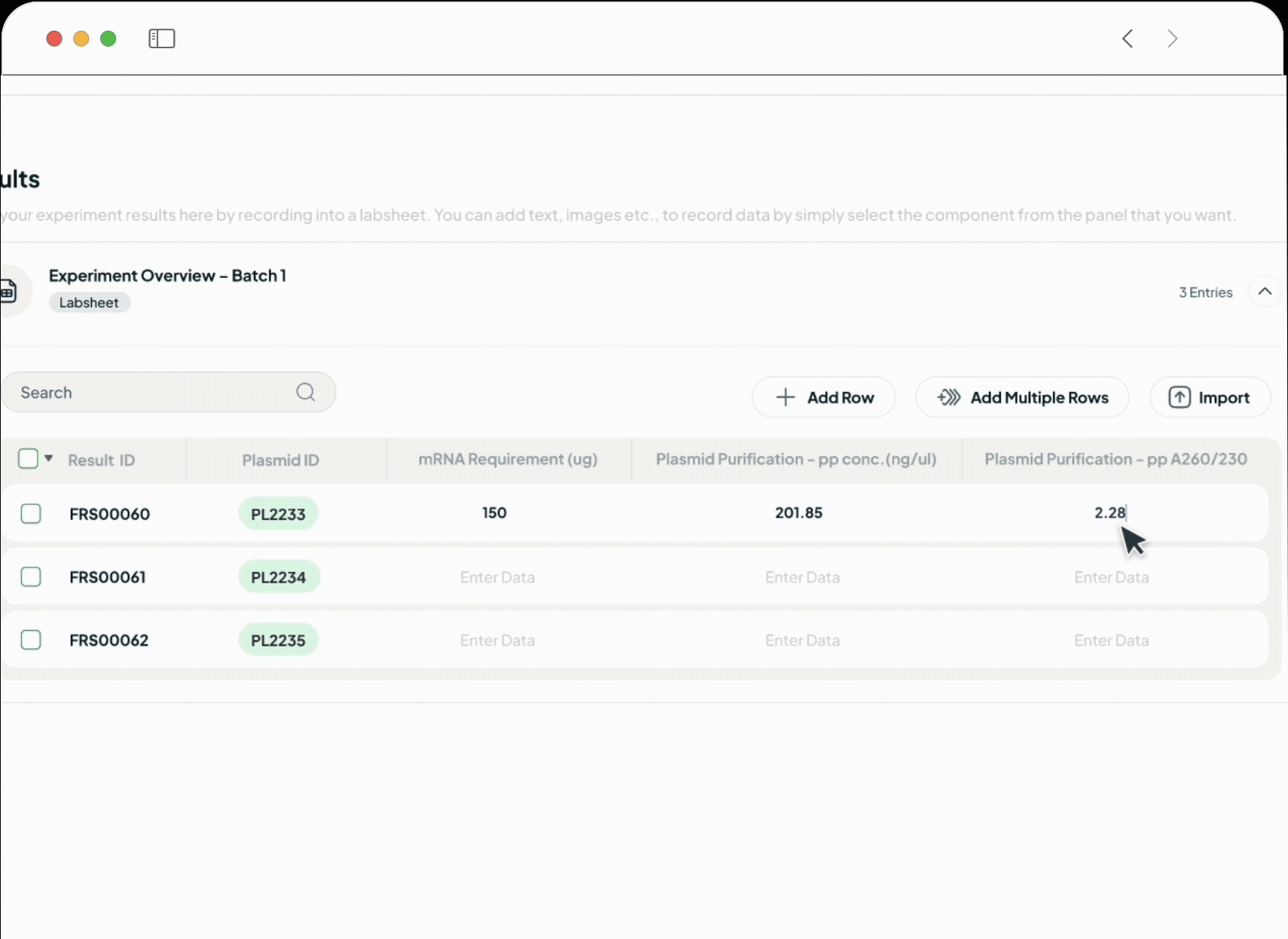
Streamline your entire product journey on one intelligent platform. We unify all your critical data—from batch records and SOPs to complaints and regulatory submissions—using the power of AI. This single source of truth fosters improved collaboration, automates routine tasks, proactively identifies risks, and ensures seamless GxP compliance. By connecting R&D with commercial operations, we empower your teams to reduce costs, improve quality, and accelerate your time to market.
Streamline your entire product journey on one intelligent platform. We unify all your critical data—from batch records and SOPs to complaints and regulatory submissions—using the power of AI. This single source of truth fosters improved collaboration, automates routine tasks, proactively identifies risks, and ensures seamless GxP compliance. By connecting R&D with commercial operations, we empower your teams to reduce costs, improve quality, and accelerate your time to market.
AI-Powered Tools for Each Phase of the Product Lifecycle
Our platform powers every phase of the life sciences lifecycle with a suite of specialized, AI-driven modules. This integrated toolkit includes our Manufacturing Execution System (MES), Quality Management System (QMS), Document Management System (DMS), Learning Management System (LMS), and a unified ELN + LIMS. Each module is built with a relentless focus on the user, featuring an intuitive interface and a seamless experience that feels natural from day one.
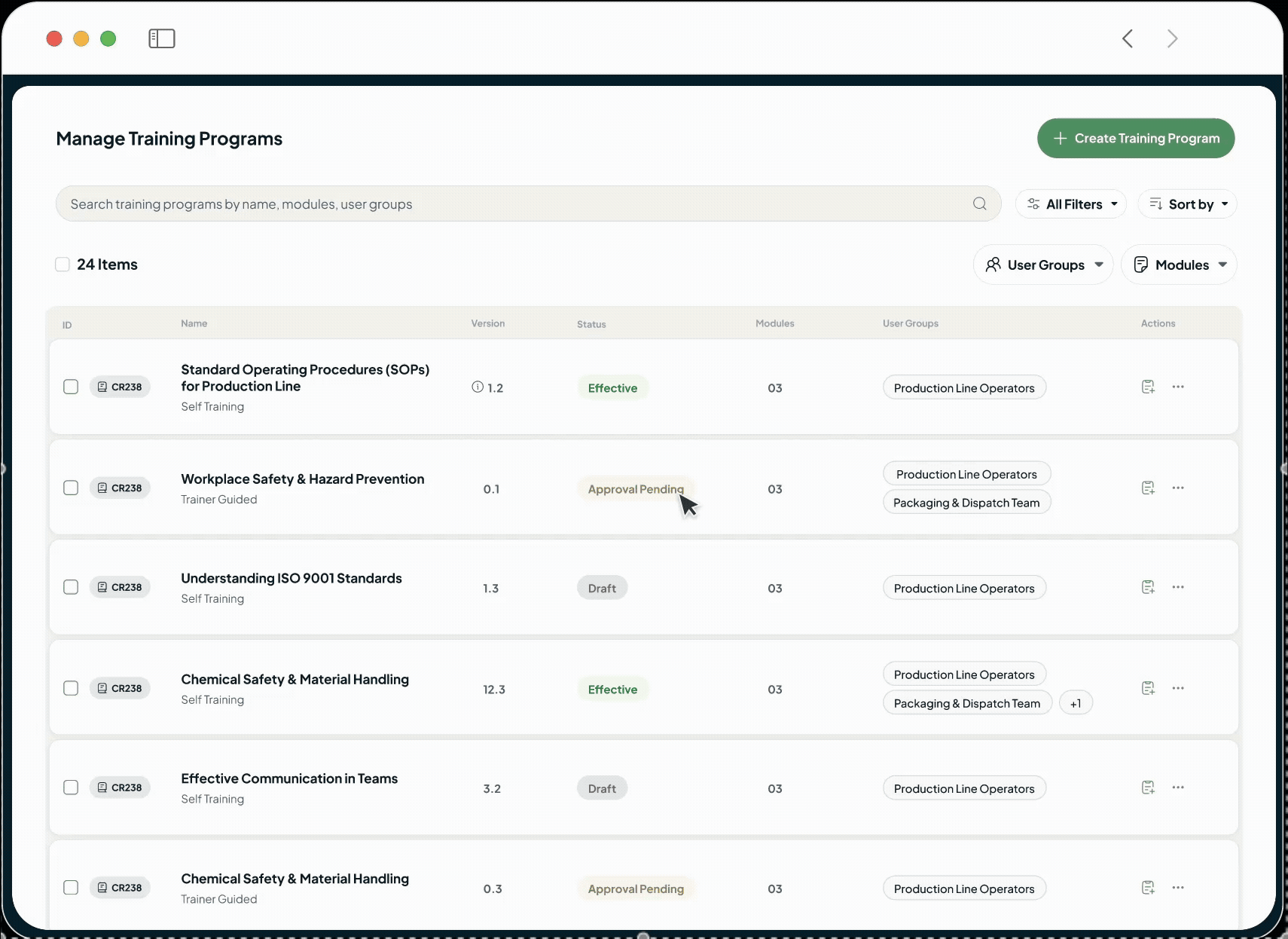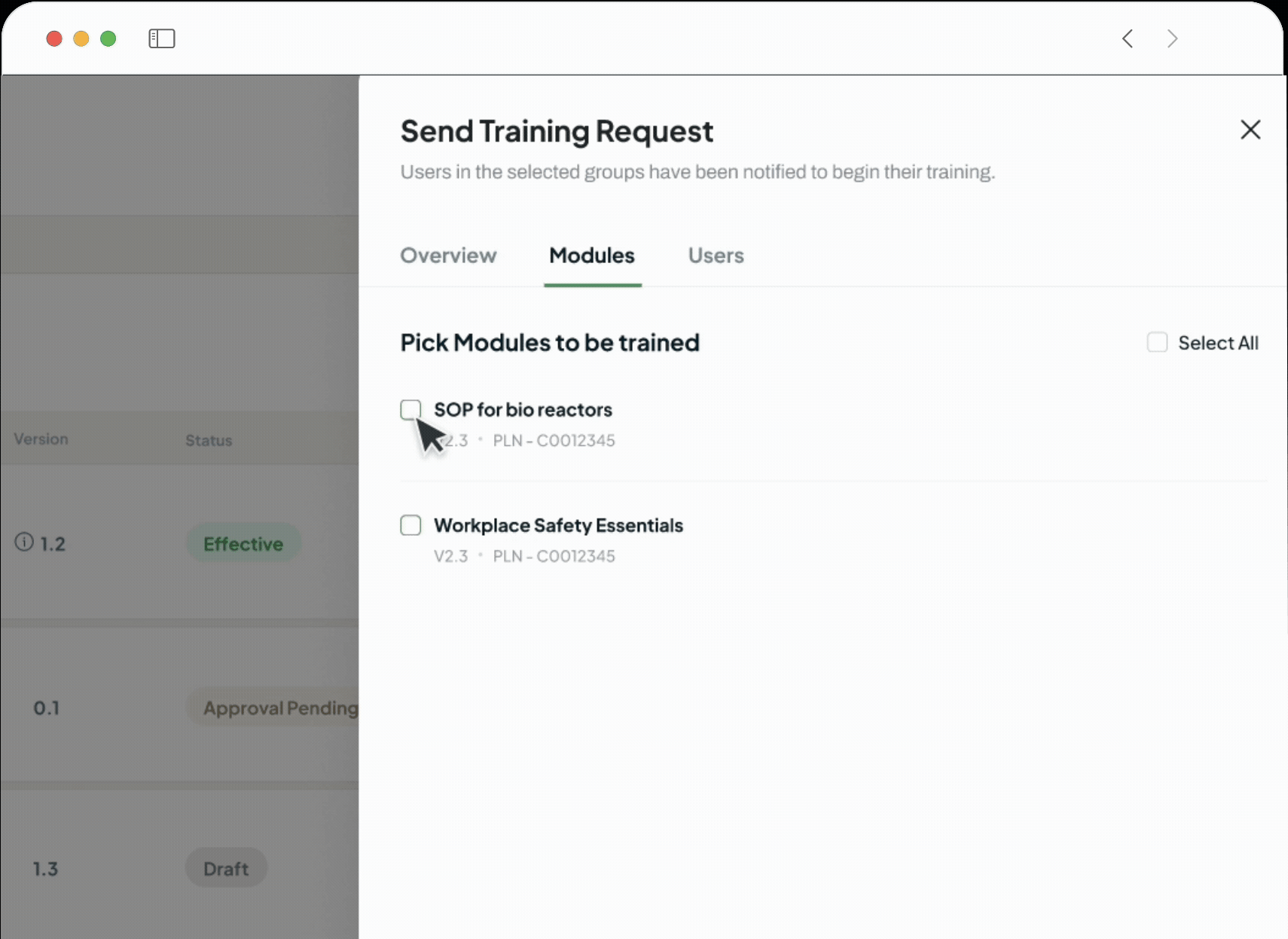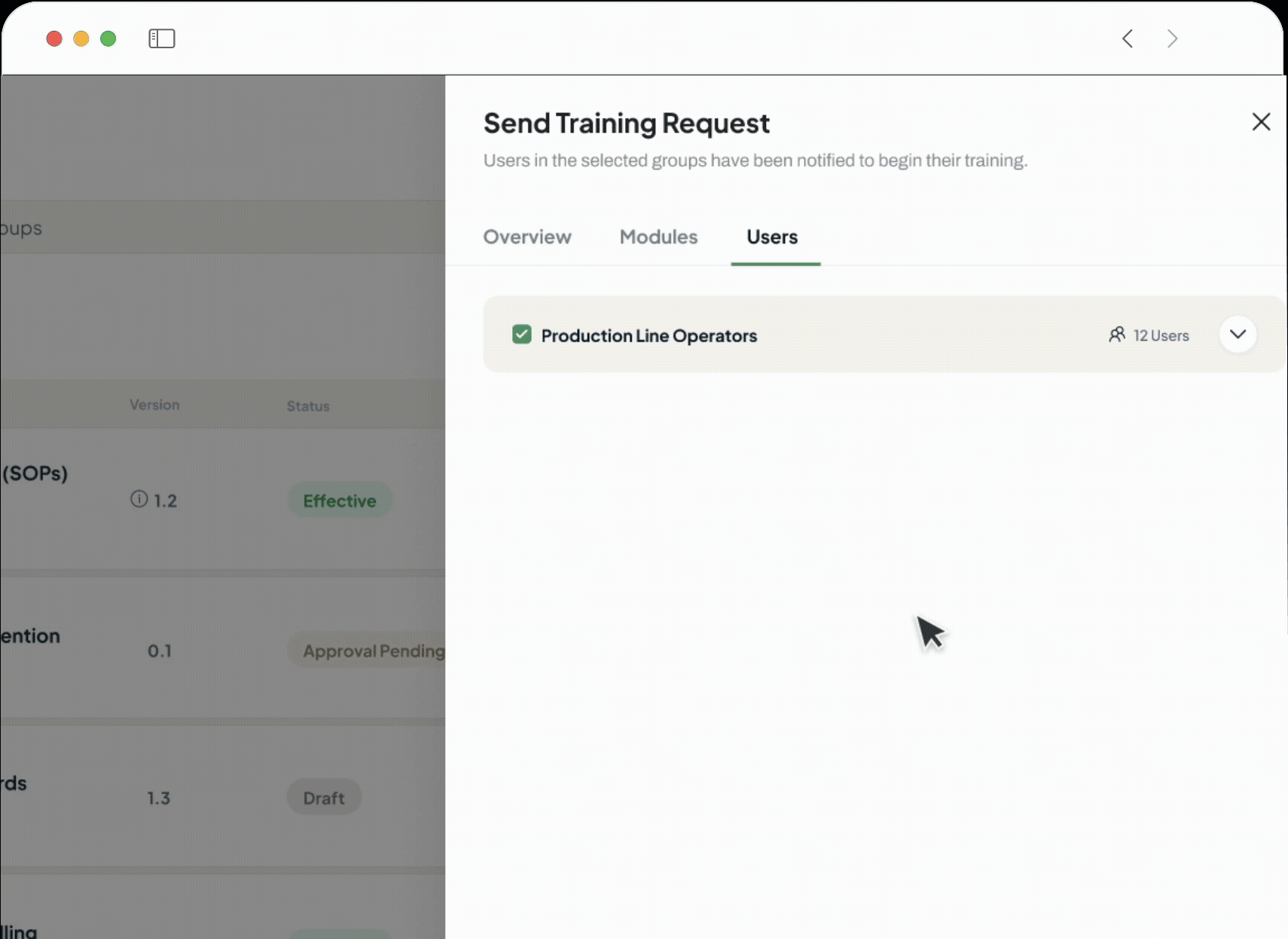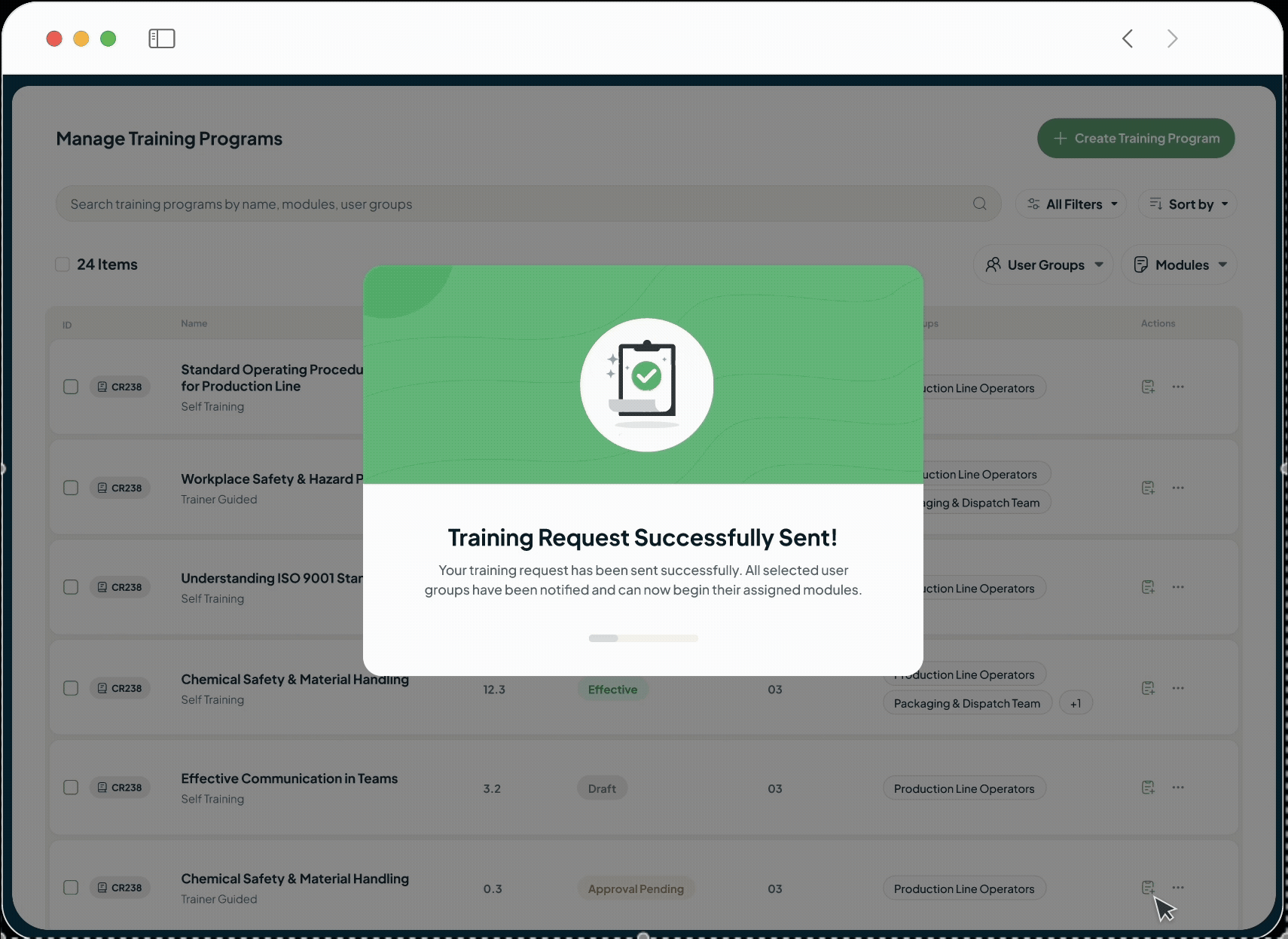
What Industry Leaders are Saying
Tailored Solutions for Every Life Sciences Sector
Our modular platform seamlessly adapts to the unique workflows of each sector — accelerating innovation, ensuring compliance, and driving operational excellence.

Bio Pharma Manufacturing


Medical Devices




CDMO / CRDMO




Cosmetics




Nutrition Supplements


Food Production


Accelerate Timelines Without Compromising Compliance
Our platform eliminates the traditional trade-off between speed and regulatory diligence. By automating critical GxP workflows and unifying your data, we make the compliant path the fastest path. This allows you to drastically shorten development and release cycles with the confidence that every action is documented and your organisation is always audit-ready.

Face Audits with Confidence
Centralize your quality processes, documents, and training records with our platform for a single, searchable source of truth. Confidently demonstrate control and answer auditor requests instantly with a complete audit trail.

Proactively Prevent Deviations
Shift from reactive firefighting to proactive quality control, saving time and money. Our system prevents human error by ensuring operators use only calibrated equipment and the latest SOPs. Digital process enforcement reduces deviations, minimizes rework, and lowers overall quality costs.

Minimize Administrative Work
Stop allowing your highly skilled experts to spend their day on low-value administrative work. Our AI-powered platform automates the repetitive tasks that consume your team's time, from authoring documents (DMS) to analyzing quality events and tracking training (QMS & LMS).
Driving Impact Across Every Milestone
100% Visibility
Gain complete visibility into manufacturing and quality data with a unified dashboard. Instantly identify issues and make informed decisions to maintain smooth operations.
Easy Collaboration
Unify manufacturing, quality, and compliance teams on one platform for a single source of truth, eliminating communication delays and speeding up approvals and issue resolution.
Reduce Cycle Times
Streamline operations with automated workflows, reducing cycle times for quality processes and batch release, thus boosting throughput and agility.
30% Quicker Batch Release
> 90%
10 - 15%
30%
Up to 3
Unify GxP Operations in One AI platform
Engineered for Simplicity, Automation and Regulatory Compliance
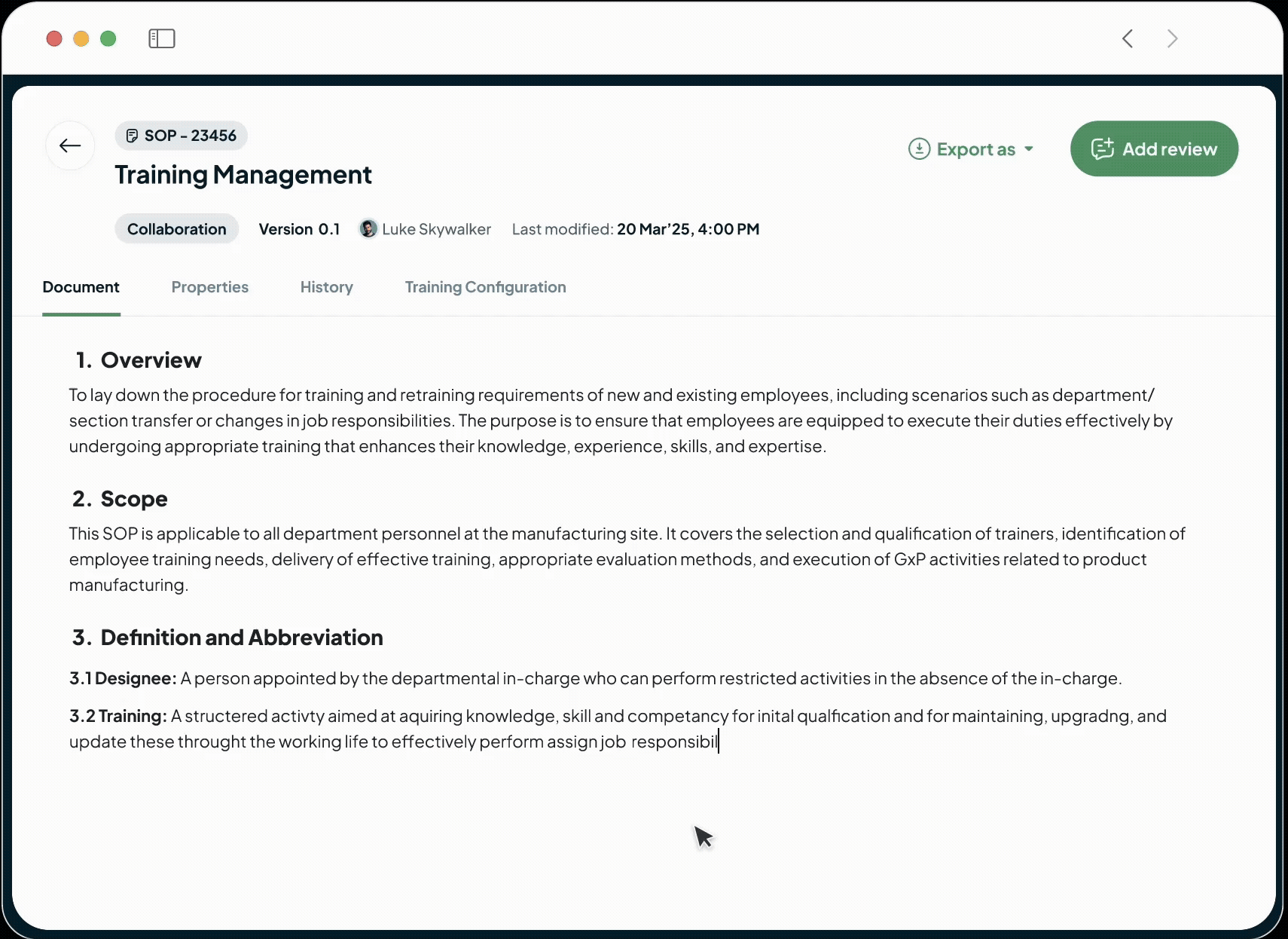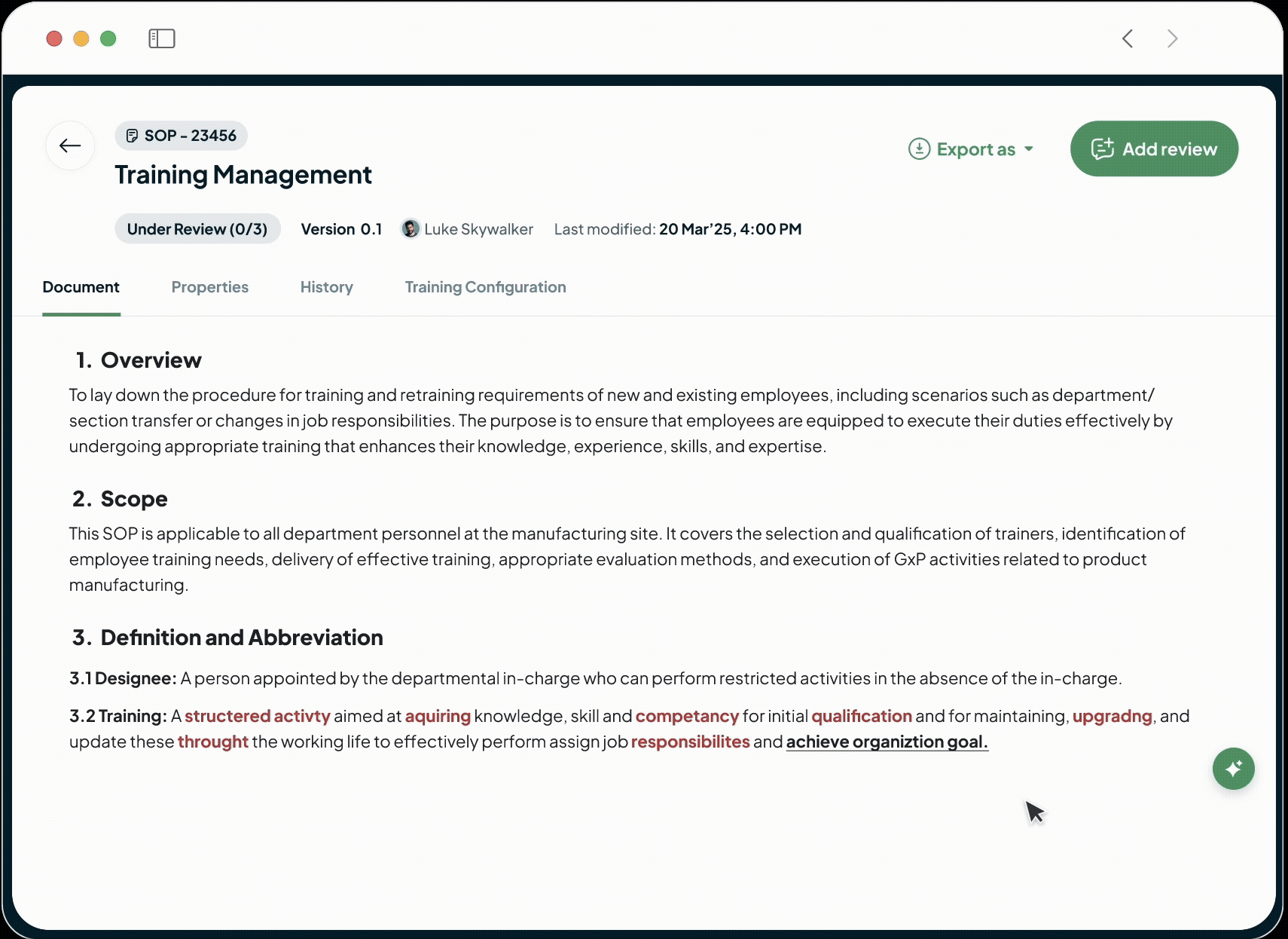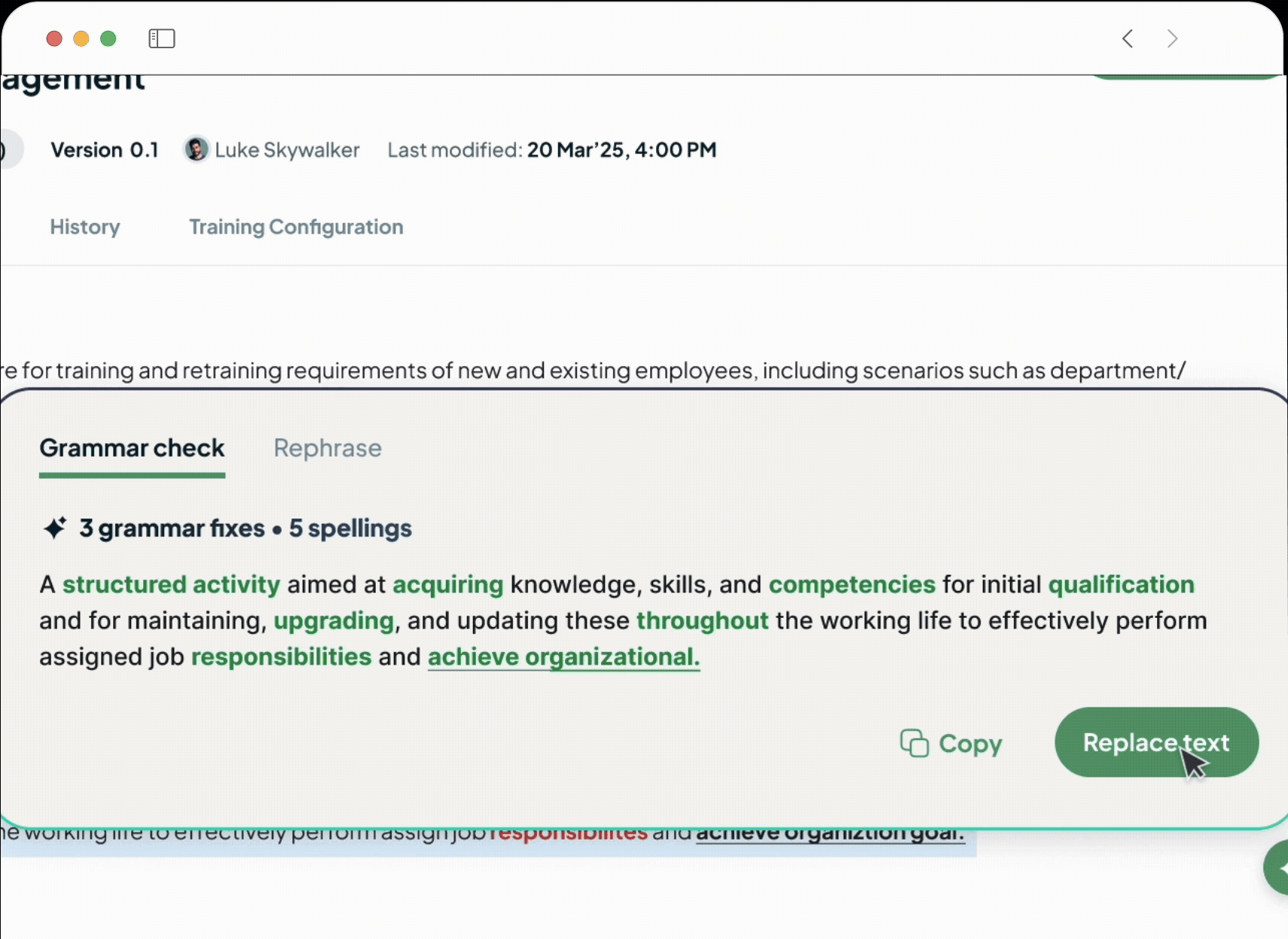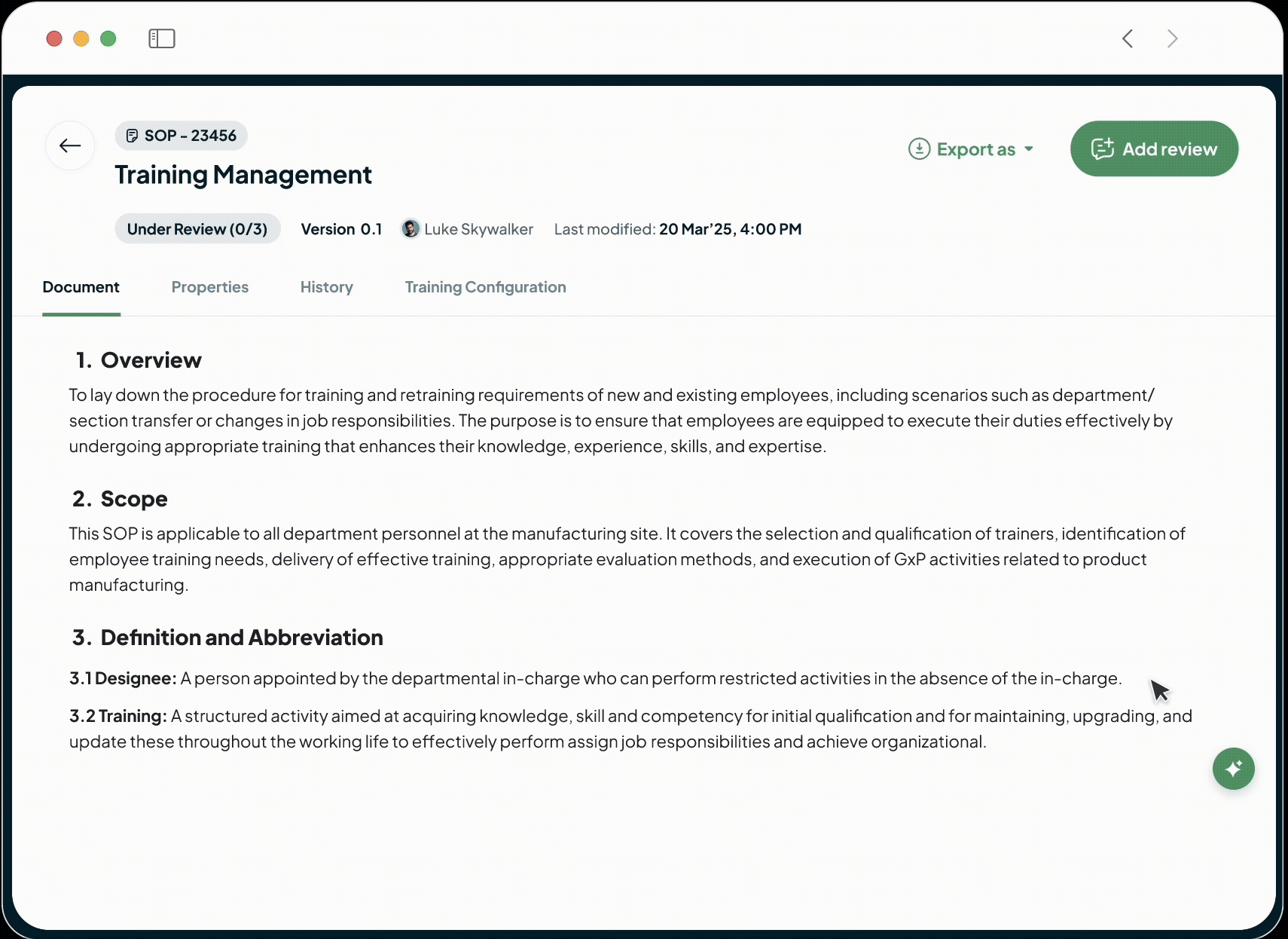
AI-First Automation
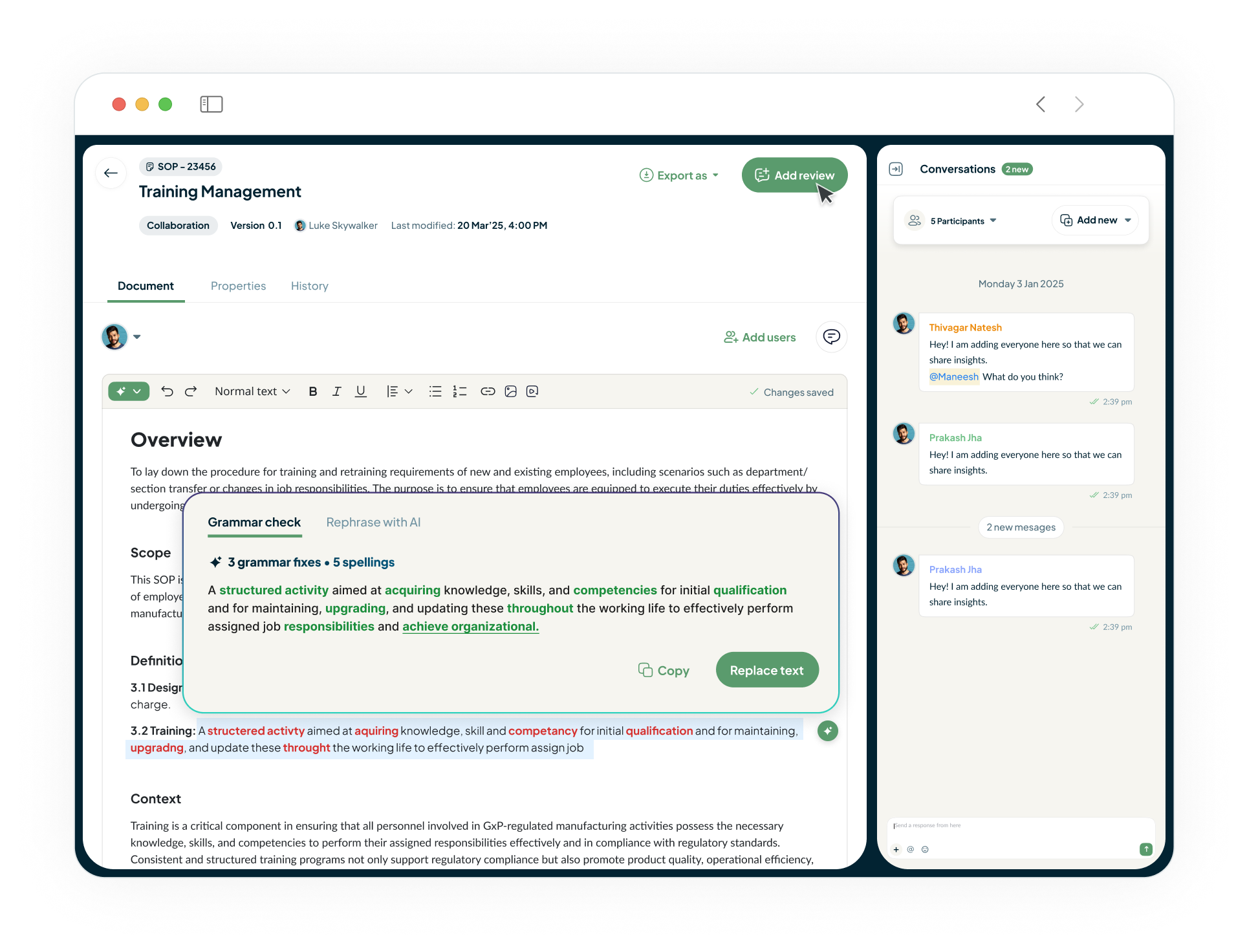
Go beyond simple automation with a suite of AI co-pilots integrated into your workflows. Effortlessly author documents, get instant answers, accelerate root cause analysis with Investigation Agents, and auto-generate training assessments.
Document Authoring
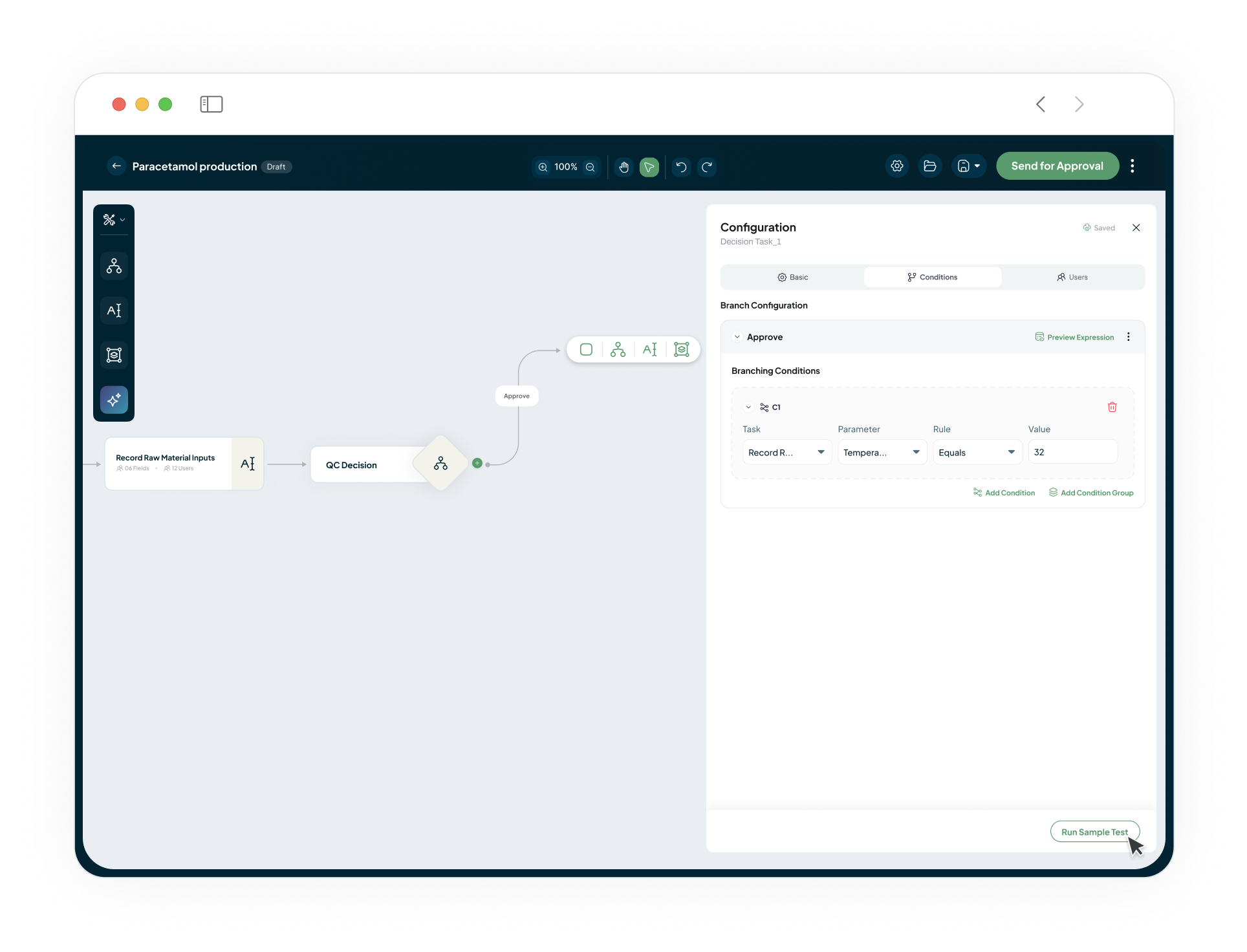
No Code Process Builder
Collaboration and Configuration
Adapt the platform to your unique operational needs without lengthy implementation. Use the no-code process builder to digitise your workflows, foster seamless teamwork with in-built conversations on any record, and design flexible review and approval cycles that match how your teams actually work.
Full Spectrum Compliance & Security
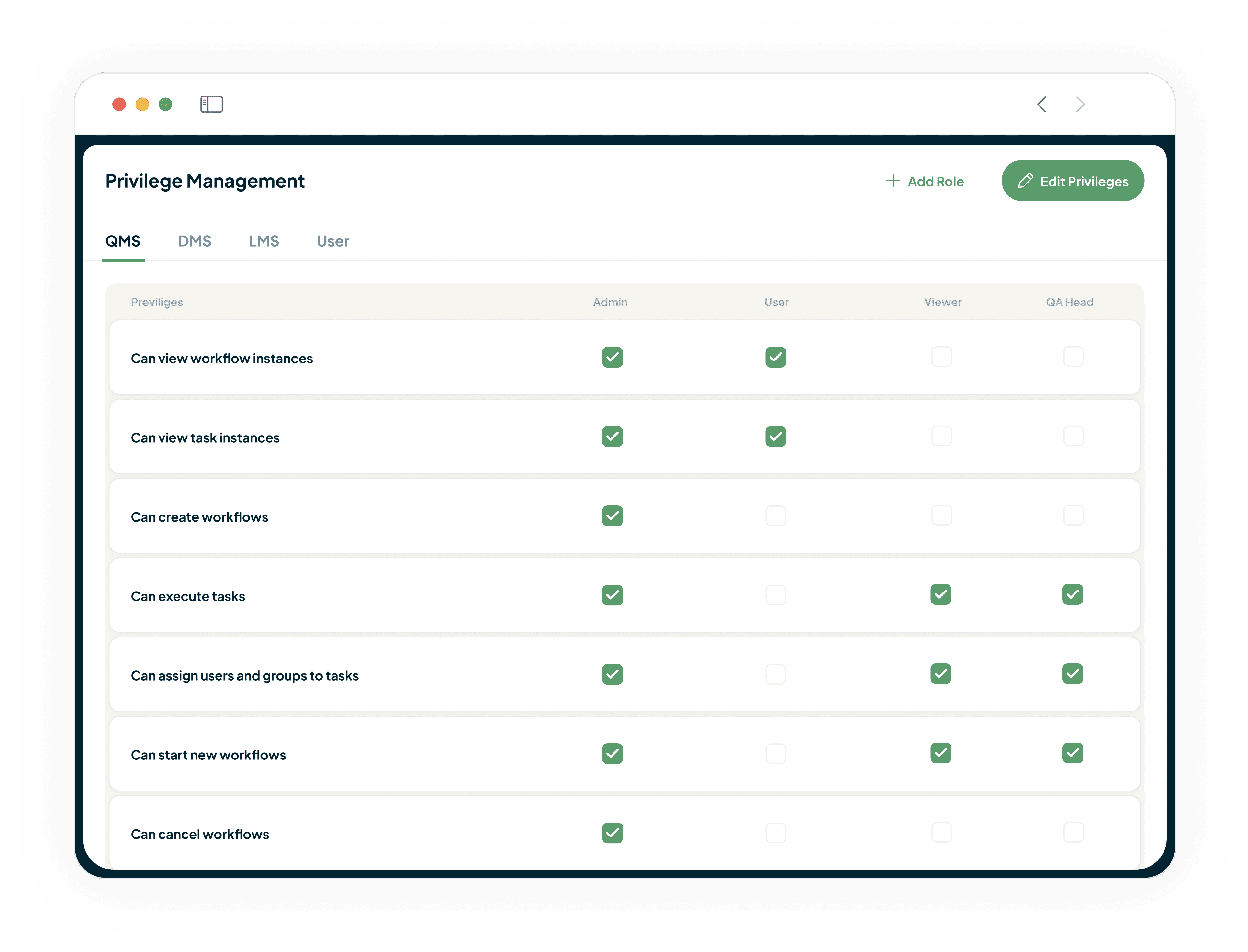
Operate with confidence on a platform built for GxP compliance software and enterprise-grade security. Every action is recorded in a comprehensive, immutable audit trail, and granular Role-Based Access Control ensures the right people have the right access, by design.
Role Based Access Control
Frequently Asked Questions
1. Is Zenopsys compliant with 21 CFR Part 11 and EU Annex 11?
2. What validation documentation is available for Zenopsys?
3. Can Zenopsys integrate with our existing technology stack?
4. How quickly can our teams go live with Zenopsys?
5. What's the pricing model of Zenopsys?
Who We Are ?
We are driven by a passion to free brilliant scientists and operators from the regulatory complexities that hold them back. Backed by venture capital and a dream team of industry advisors, we are building the future of intelligent, effortless compliance, ensuring companies can bring life-changing products to market faster and more safely than ever before.















